In the pantry theres vinegar and baking soda an acid and a base. Acids usually have a sour taste like lemon juice and will burn your skin.
Acid and bases for dummies.
Acids and bases for dummies. Part of Chemistry For Dummies Cheat Sheet A lot of chemistry requires you to understand the difference between acids and bases. An acid is a substance that donates an H ion to another chemical species called a base. A base is a substance that accepts combines with an H ion.
Acids usually have a sour taste like lemon juice and will burn your skin. Bases usually have a bitter taste and feel slippery like detergent. Chemically speaking an acid is a compound that gives away or releases a hydrogen ion H during a chemical reaction and a base is a compound that captures a hydrogen ion during a chemical reaction.
The slight polarity of a water molecule makes it a powerful agent for dissolving acid and base compounds into liquid form or solution. Acids bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other.
A base that can dissolve in water is also called an alkali. Science and technology makes good use of acids and bases. Car batteries use a strong acid called sulphuric acid.
Chemical reactions between the acid and lead plates in the battery help make electricity to start the car. They are also used in many household cleaning products baking soda and to make fertilizer for crops. Acids and bases can help neutralize each other.
Acids turn litmus paper red bases turn it blue. Strong bases can be slippery and slimy feeling. Definition of Acid and Base.
An acid is a proton H donor also accepts electron pairs and produce hydrogen ions H Base. A base receives protons also are electron pair donors and finally produce hydroxide ions OH- Alkali. An alkali is a soluble base.
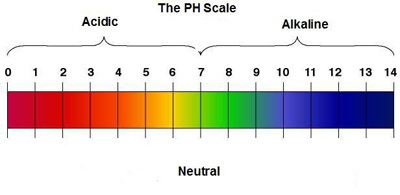
All alkalies are bases but not all bases are alkalies. The best and the biggest channel for science videos for kidsKids can learn about acids and bases in this educational videoThis is an animated lesson for pr. Label the pH scale.
Have the kids label each section of the scale with a number in consecutive order with 0 at the bottom and 14 at the top. Write Acids near the bottom and Bases at the top. Explain that numbers 0-69 apply to acids 7 is neutral and 71-14 refer to bases.
Wash burette with HCL acid so that the burette is coated with acid. Put burette on a clamp stand. Wash pipette pump with sodium hydroxide so that the pipette pump is coated with base for the most accurate results.
Put funnel on top of the burette. Pour around 30 mL of HCL depending on the size of the burette in the burette. This is all Acid-Base balance means.
ACIDIC - TOO MUCH ACID - Lower than 735 means that there is too much acid in your system. NORMAL - Just right. PH between 735 and 745 is perfect for homeostasis.
ALKALINE - NOT ENOUGH ACID - It just means NOT acidic enough. PH higher than 745 is considered Alkaline. Acid and Bases for Dummies.
PH pOH H and OH- QuizTest. What is a buffer. A solution that resists changes in pH when acid or alkali is added to it.
This means that the buffer does not change pH with the proper acid and base combination. In the pantry theres vinegar and baking soda an acid and a base. Peek under the sink and youll notice the ammonia and other cleaners most of which are bases.
Check out that can of lye-based drain opener its highly basic. In the medicine cabinet youll find aspirin an acid and antacids of all types. Start superscript plus end superscript.
The pH scale is often said to range from 0 to 14 and most solutions do fall within this range although its possible to get a pH below 0 or above 14. Anything below 70 is acidic and anything above 70 is alkaline or basic. Acids can be neutralized too here are the equations for acids.
Acid metal – salt hydrogen gas. Acid carbonate – salt H20 C02. Acid alkali – salt H20.
Acid base – salt H20. ACIDS AND BASES -Acid. A compound that produces hydronium ions when dissolved in water.
-All acids have certain chemical properties that are similar. Foods that taste sour often contain acids. Lemons apples oranges for example all contain citric acid.
Dairy products that have spoiled contain butyric acid. An acid is a substance that donates an h ion to another chemical species called a base. Acids can be neutralized too here are the equations for acids.
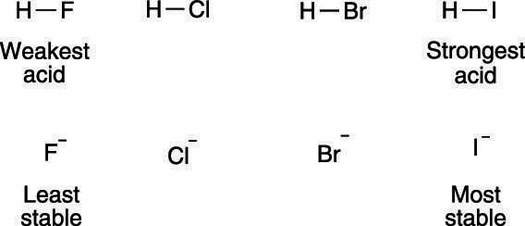
Science and technology makes good use of acids and bases. The pka value of an acid is a quantitative measurement of a molecule s acidity. Acid and bases for dummies.
Learn the basics about what salts are as part of the overall topic of acids and basesSUBSCRIBE to the Fuse School YouTube channel for many more educational. Acid and Bases for Dummies. PH pOH H and OH- QuizTest Glossary.
Properties of acid Conduct Electricity Neutralizes bases Tastes sour Turns blue litmus paper to red Keeps red litmus paper red Corrosive pH is less than 7 When neutralized with a base they produced salt and water.