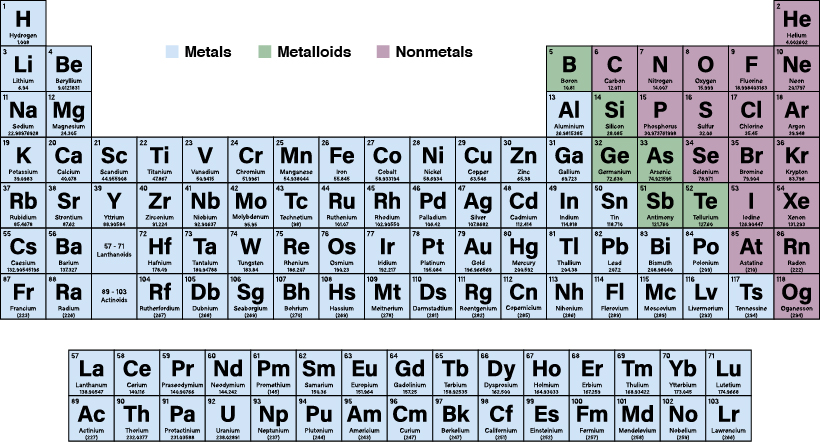
These elements have similar chemical properties that differ from the elements considered metals. Interactive periodic table with up-to-date element property data collected from authoritative sources.
Good conductor of heat.
All metals in the periodic table. The metals share several common properties including. Solid at room temperature with the exception of mercury usually shiny. Good conductor of heat.
Good conductor of electricity. Malleable able to be pounded into sheets. List of Elements Alkali Metals.
Alkali metals are in group IA on the far left side of the periodic table. They are highly reactive. The alkaline earth metals are found in group IIA of the periodic table which is the second.
They conduct heat and. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po. There are only two exceptions ie two elements in that sequence between number 5 and number 84 that are not metals.
Atomic number 32 Germanium Ge. And atomic number 52 Antinomy Sb. Every element is either a metal or a non-metal.
All metals are situated on the left hand side of the periodic table and all the non-metals are on the right. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right. In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84.
Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals. All the different elements are arranged in a chart called the periodic table. A Russian scientist called Dmitri Mendeleev produced one of the first practical periodic tables in the 19th century.
The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. An extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven up through oganesson which completes the seventh period row in the periodic table at atomic number Z 118As of 2021 no element with a higher atomic number than oganesson has been successfully synthesized.
All elements in the eighth period and beyond thus remain. Hence Gold is the most malleable metal in the periodic table. Become a member and unlock all Study Answers.
Try it risk-free for 30 days Try it risk-free Ask a question. Our experts can answer. Interactive periodic table with up-to-date element property data collected from authoritative sources.
Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game. IE decreases going down a column of the periodic table and increases from left to right in a row. Thus alkali metals have the lowest IE in a period and Rare gases have the highest.
Other resources related to the Periodic Table. List of Periodic Table Elements in Hebrew. Chemical Evolution of the Universe.
The first person to come up with a periodic table of elements was Dmitri Ivanovich Mendeleev a Russian chemist. He came up with the first version of periodic table in 1864. Mendeleevs table was based on the atomic weight of the elements.
He found that there were many elements that shared similar properties and occurred periodically. The nonmetals or non-metals are a group of elements located on the right side of the periodic table except for hydrogen which is on the top left. These elements are distinctive in that they typically have low melting and boiling points dont conduct heat or electricity very well and tend to have high ionization energies and electronegativity values.
The nonmetal elements occupy the upper right-hand corner of the periodic table. Nonmetals include the nonmetal group the halogens and the noble gases. These elements have similar chemical properties that differ from the elements considered metals.
The nonmetal element group is a subset of these elements. The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. In the periodic table the vertical columns are called groups and the horizontal rows are called periods.
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley which states that the properties of.