The period number is the same as the number of. All the members of a family of elements have the same number of valence electrons and similar chemical properties.

The periodic table has seven He horizontal rows.
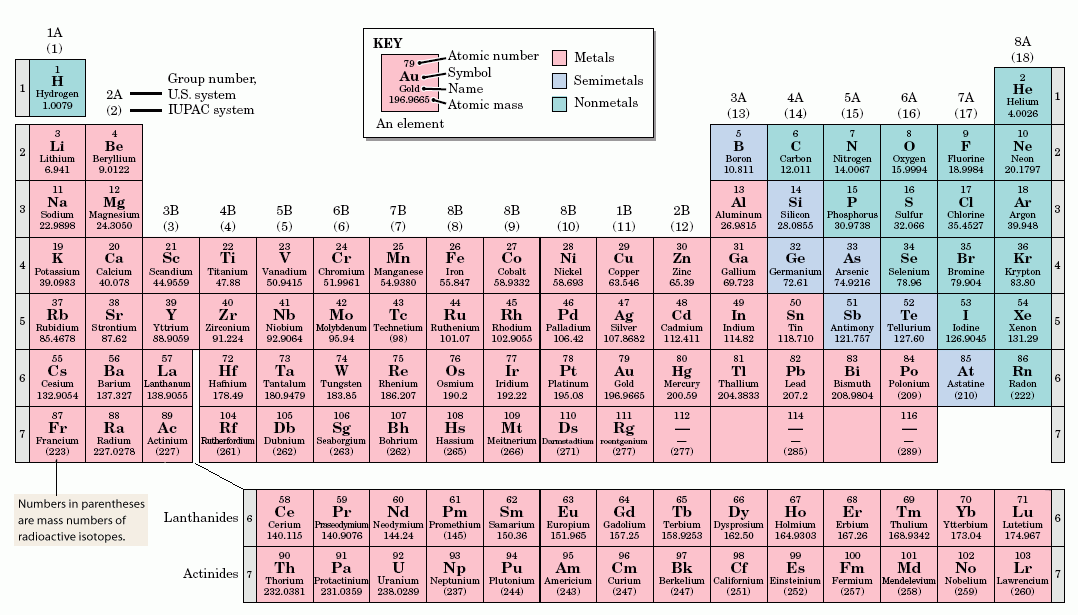
Horizontal rows in the periodic table are called. On the periodic table the seven horizontal rows are called periods. On the left-hand side of the periodic table the row numbers are given as one through seven. Moving across a period from left to right the atomic number of the elements increases.
Rows six and seven contain elements called transition elements which are subdivided into the actinide and lanthanide series. According to the Aufbau principle the order of filling atomic orbitals is 1s 2s 2p 3s 3p and so on. The elements in a modern periodic table are arranged in an increasing order of their atomic numbers.
In the modern periodic table the horizontal rows are known as periods and vertical columns are known as groups. The horizontal rows on the periodic table of the elements are called periods. Every element in a period has the same number of atomic orbitals.
For instance hydrogen and helium are in the first period so they both have electrons in one orbital. Rows in the periodic table periods. Elements in the same horizontal row are in the same period.
The periods are numbered from top to bottom. The period number is the same as the number of. Horizontal rows in periodic table are called periods they are seven in numbers elements in same period may not have similar quality.
While vertical rows in periodic table are called columns or family. The modern periodic table is based closely on the ideas he used. The elements are arranged in order of increasing atomic number.
The horizontal rows are called periods. How many horizontal rows are there in the modern periodic table and what are they called. Asked Jun 13 2018 in Class X Science by aditya23 -2145 points 0 votes.
The modern periodic table was constructed by the Russian chemist Dmitri Mendeleyev in 1869. The modern periodic table consists of horizontal rows called periodsand vertical columns called groups. These are discussed below.
Groups in Modern Periodic Table. A group may be defined as vertical column in the periodic table. In terms of electronic.
The horizontal rows of elements in the periodic table are called periods therefore the answer is B. The periods are numbered 1 through 7. A vertical column of elements in the periodic table is called a group A horizontal row of elements in the periodic table is called a period Atoms of elements in the same group have the same numbers of valence electrons As you move from left to right on the periodic table the atomic number increases.
The horizontal rows are called periods and the vertical columns are called groups. This video explains the pattern of the periodic table in more detail. A look at why the periodic table is.
View Screenshot 2021-02-15 at 42653 PMpng from CHEM SCH4U at Eastwood Collegiate Institute. Periods and Chemical Families 2. The periodic table has seven He horizontal rows.
- Each of these. The Periodic table is arranged in ascending order based on the atomic numbers of elements which is equal to the protons in their cores. The horizontal rows of the Periodic table are called periods.
The elements in a given row have the same number of layers in their electron shells. The vertical columns of the Periodic table are called. All the different elements are arranged in a chart called the periodic table.
The elements are arranged in order of increasing atomic number. The horizontal rows are called. The horizontal rows in the periodic table are periods while the vertical rows are called groups.
What are the horizontal rows called in the periodic table. Closer to farther from. Electrons that are closer tofarther from the nucleus have less energy than those electrons that are closer tofarther from the nucleus.
Horizontal rows on a periodic table are called periods. Vertical columns on a periodic table are called groups. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior.
All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods.