Informative Awnsers Teaching About Atoms And The Periodic Table Try Using These Cornell Format Doodle Notes Which Provide Structure Science Notes Teaching Chemistry Doodle Notes - The physical and chemical properties of the elements are a periodic function of their atomic masses. Periodic table consist of horizontal rows and vertical column.
All the elements in a period have valence electrons in the same shell.
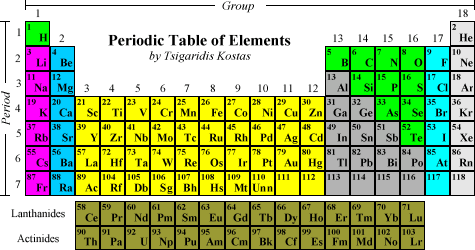
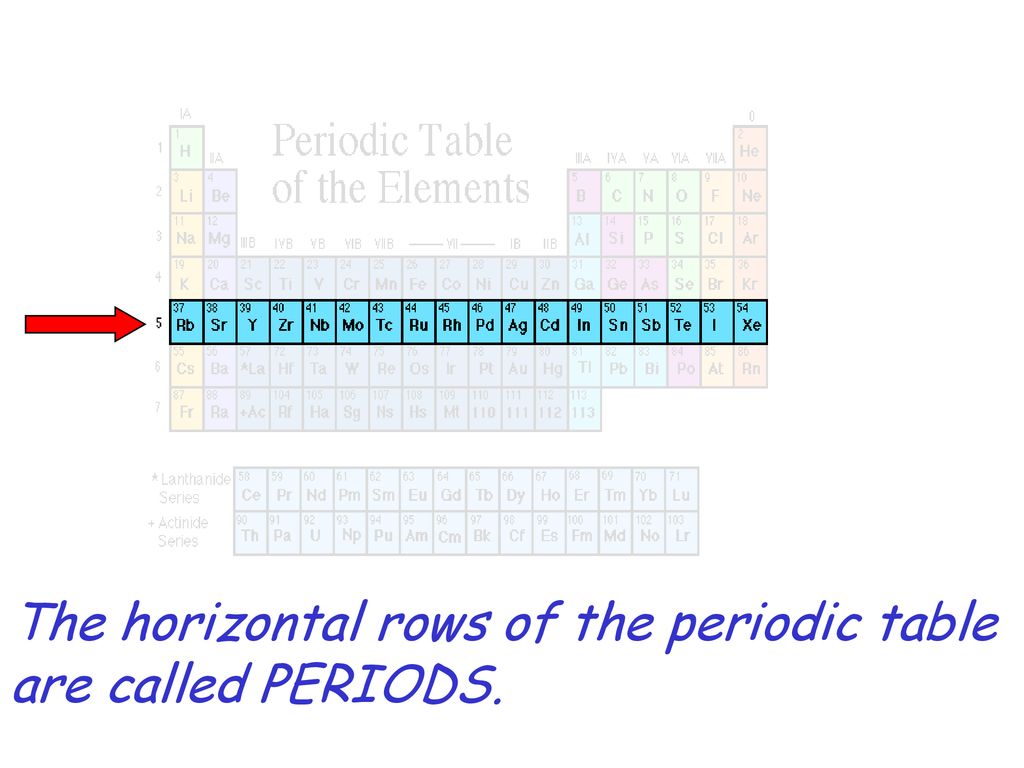
Horizontal rows of the periodic table are called. On the periodic table the seven horizontal rows are called periods. On the left-hand side of the periodic table the row numbers are given as one through seven. Moving across a period from left to right the atomic number of the elements increases.
Rows six and seven contain elements called transition elements which are subdivided into the actinide and lanthanide series. According to the Aufbau principle the order of filling atomic orbitals is 1s 2s 2p 3s 3p and so on. The elements in a modern periodic table are arranged in an increasing order of their atomic numbers.
In the modern periodic table the horizontal rows are known as periods and vertical columns are known as groups. The horizontal rows of elements in the periodic table are called periods therefore the answer is B. The periods are numbered 1 through 7.
Become a member and unlock all Study Answers Try it. Rows in the periodic table periods. Elements in the same horizontal row are in the same period.
The periods are numbered from top to bottom. The period number is the same as the number of. A Russian scientist called Dmitri Mendeleev produced one of the first practical periodic tables in the 19th century.
The modern periodic table is based closely on the ideas he used. A vertical column of elements in the periodic table is called a group. A horizontal row of elements in the periodic table is called a period.
Atoms of elements in the same group have the same numbers of valence electrons. As you move from left to right on the periodic table the atomic number increases. 3 numbers of valence electrons.
W Norton Compan The Modern Periodic Table Periodic table Horizontal rows are from CHEM 114 at University of Saskatchewan. Periodic Table And Element Structure. Informative Awnsers Teaching About Atoms And The Periodic Table Try Using These Cornell Format Doodle Notes Which Provide Structure Science Notes Teaching Chemistry Doodle Notes - The physical and chemical properties of the elements are a periodic function of their atomic masses.
This is essentially an abbreviation of the elements name. How many horizontal rows are there in the modern periodic table and what are they called. Asked Jun 13 2018 in Class X Science by aditya23 -2145 points 0 votes.
Horizontal rows on a periodic table are called periods. Vertical columns on a periodic table are called groups. Periods on the Periodic Table.
The rows on the periodic table are called periods. All the elements in a period have valence electrons in the same shell. The number of valence electrons increases from left to right in the period.
When the shell is full a new row is started and the process repeats. Horizontal rows in the periodic table are called periods. Also access detailed answers to various other Science Maths questions for free.
Periodic table consist of horizontal rows and vertical column. The horizontal rows are called period while vertical column are called groups. There are 7 periods and 18 column in modern periodic table.
Here we will discuss some trends along period and groups. The Periodic table is arranged in ascending order based on the atomic numbers of elements which is equal to the protons in their cores. The horizontal rows of the Periodic table are called periods.
The elements in a given row have the same number of layers in their electron shells. The vertical columns of the Periodic table are called. In rows 4 through 7 there is a wide central section containing elements each of which is called a _____ transition element Rows 6 and 7 also contain two other sets of elements that are listed below the main chart.
In the periodic table there are 18 columns and 7 rows. The columns are known as the groups which are grouped together to form a block like s-block p-block etc. According the valence electrons.
The Horizontal Rows Of Periodic Table Are Called. By Review Home Decor October 15 2018. The horizontal rows on a periodic table periodic table of elements with worked in the periodic table how many columns periodic table guide shmoop.
The Horizontal Rows Of Periodic Table Are Called.