
To find the limiting reagent take the moles of each substance and divide it by its coefficient in the balanced equation. If youre seeing this message it means were having trouble loading external resources on our website.
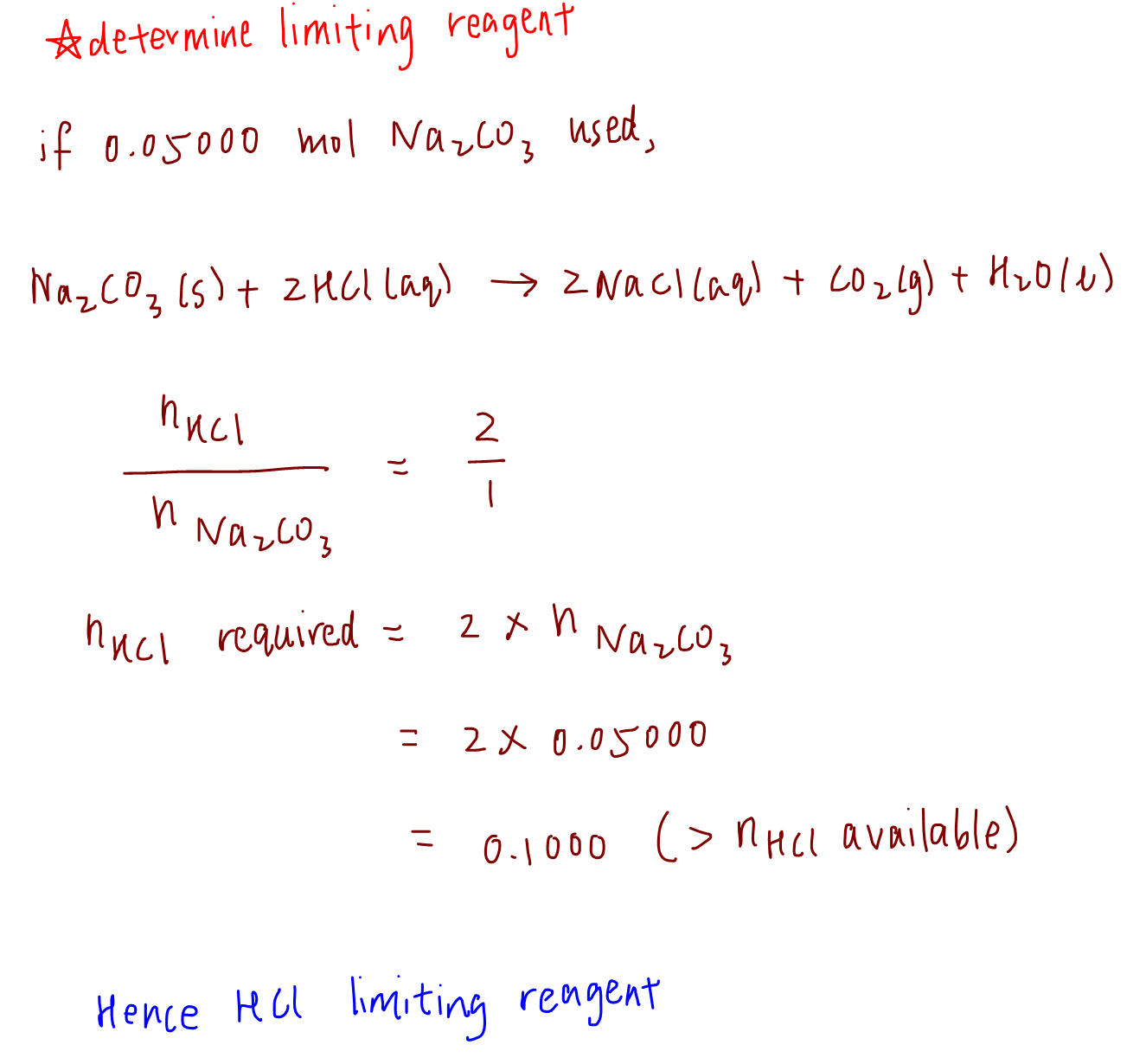
Another method is to calculate the grams of products produced from the quantities of reactants in which the reactant which produces the smallest amount of product is the limiting reagent.
How to calculate limiting reagent. Grams H 2 O 108 grams O 2 O. Much more water is formed from 20 grams of H 2 than 96 grams of O 2. Oxygen is the limiting reactant.
After 108 grams of H 2 O forms the reaction stops. To determine the amount of excess H 2 remaining calculate how much H 2 is needed to produce 108 grams of H 2 O. So heres the solution.
Before doing anything else you must have a balanced reaction equation. Dont waste good thought. Determine the limiting reagent if 100 g of ammonia and 100 g of oxygen are present at the beginning of the reaction.
Identify the excess reagent. The following points should be considered while attempting to identify the limiting reagent. When there are only two reactants write the balanced chemical equation and check the amount of reactant B required to.
The reactant which is in a lesser amount than is required by stoichiometry is the. Calculate the molecular weight of each reactant and product. You will need to know these numbers to do yield calculations.
To calculate the molecular weight of a molecule simply add up the masses of the individual atoms. Determine the balanced chemical equation for the chemical reaction. The balanced chemical equation is already.
Convert all given information into moles most likely through the use of molar mass as a conversion factor. Calculate the mole ratio from the. Final words The limiting reagent or reactant in a reaction is found by calculating the amount of product produced by each reactant.
The reactant that produces the least amount of product is the limiting reactant. Find the Limiting Reactant Example Question. Ammonia NH 3 is produced when nitrogen gas N 2 is combined with hydrogen gas H 2 by the reaction N 2 3 H 2 2 NH 3 50 grams of nitrogen gas and 10 grams of hydrogen gas are reacted together to form ammonia.
Learn how to identify the limiting reactant in a chemical reaction and use this information to calculate the theoretical and percent yields for the reaction. If youre seeing this message it means were having trouble loading external resources on our website. Enter any known value for each reactant the limiting reagent will be highlighted.
Use uppercase for the first character in the element and lowercase for the second character. Ionic charges are not yet supported and will be ignored. Replace immutable groups in.
A The limiting reagent is the compound that will be depleted first and thus determine the amount of product that will form. This can be determined from the following ratio. You find the limiting reagent using the balanced equation.
Yours tells you 4 moles NaAlO2 needs 4 moles of Na2SiO35H2O to react. Simplifying this that is one mole of each You have mols of. There are two ways for how to calculate limiting reagent.
One method is to find and compare the mole ratio of the reactants that are used in the reaction. Another method is to calculate the grams of products produced from the quantities of reactants in which the reactant which produces the smallest amount of product is the limiting reagent. To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button.
The reactants and products along with their coefficients will appear above. Enter any known value for each reactant. The limiting reagent will be highlighted.
Use uppercase for the first character in the element and lowercase for the second character. Limiting Reactant and Theoretical Yield Problem You are given the following reaction. 2 H 2 g O 2 g 2 H 2 O l.
To find the limiting reagent take the moles of each substance and divide it by its coefficient in the balanced equation. The substance that has the smallest answer is the limiting reagent. Youre going to need that technique so remember it.
By the way did you notice that I bolded the technique to find the limiting reagent. To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button. The reactants and products along with their coefficients will appear above.
Enter any known value for each reactant. The limiting reagent will be highlighted. To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button.
The reactants and products along with their coefficients will appear above. Enter any known value for each reactant. The limiting reagent will be highlighted.
Hazel shows you how to determine the limiting reagent in a reaction and how to use mole calculations to work out which reagent is in excess.