For example K 1 is called the potassium ion just as K is called the potassium atom. The lattice is formed because the ions attract each other.

Jot down the formula of the ionic compound.
How to name ionic compounds. Naming Basic Ionic Compounds. Consult the periodic table of elements. When naming ionic compounds all of the information youll need is on the periodic table.
Jot down the formula of the ionic compound. Lets say the ionic compound youre working with is NaCl. Use a pen or.
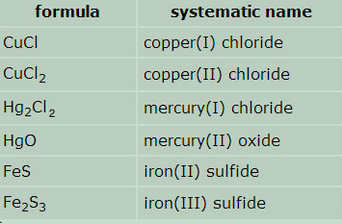
Naming Ionic Compounds Using -ous and -ic. Although Roman numerals are used to denote the ionic charge of cations it is still common to see and use the endings -ous or -ic. These endings are added to the Latin name of the element eg stannousstannic for tin to represent the ions with lesser or greater charge respectively.
The Roman numeral naming convention has wider appeal because many ions have more than two valences. An ionic compound is named first by its cation and then by its anion. The cation has the same name as its element.
For example K 1 is called the potassium ion just as K is called the potassium atom. The anion is named by taking the elemental name removing the ending and adding ide. Some examples of names for ionic compounds.
When you name ionic compounds you write the name of the metal first and then the nonmetal. Suppose that you want to name the compound that results from the reaction of lithium and sulfur. You first write the name of the metal lithium and then write the name of the nonmetal adding an -ide ending so that sulfur becomes sulfide.
If the first element you see in the compound is found on the left side of the periodic table ie. Left of the metalloid staircase it is a metal and the compound is ionic When naming ionic compounds we never indicate the ratio of component elements in the name - that is for covalent compounds only. NaCl Sodium Chloride.
Ionic and molecular compounds are named using somewhat-different methods. Binary ionic compounds typically consist of a metal and a nonmetal. The name of the metal is written first followed by the name of the nonmetal with its ending changed to ide.
For example K 2 O is called potassium oxide. An ionic compound is named first by its cation and then by its anion. The cation has the same name as its element.
For example K 1 is called the potassium ion just as K is called the potassium atom. The anion is named by taking the elemental name removing the ending and adding -ide. Ionic compounds - AQA.
An ionic compound is made up of charged particles called ions. It has a giant lattice structure with strong electrostatic forces of attraction. Ionic compounds - AQA.
An ionic compound is made up of charged particles called ions. It has a giant lattice structure with strong electrostatic forces of attraction. In this tutorial we will be learning how to name ions and ionic compounds from the formula and how to find the formula from the compound name.
This lesson will demonstrate how to write a chemical formula for a binary ionic compound when given the systemic name. When you name binary ionic compounds with transition metals the rules are the same as those of binary ionic compounds. The ionic lattice An ionic compound is a giant structure of ions.
The ions have a regular repeating arrangement called an ionic lattice. The lattice is formed because the ions attract each other. Ions of either variety may contain either a single element or more than one element.
When an ion consists of more than one element we refer to it as a polyatomic ion To recognize an ionic compound look for the presence of a metal or a known polyatomic ion - once you find one you more than likely have an ionic compound. For binary ionic compounds ionic compounds that contain only two types of elements the compounds are named by writing the name of the cation first followed by the name of the anion. For example KCl an ionic compound that contains K and Cl- ions is named potassium chloride.
Google Classroom Facebook Twitter. The names for transition metal compounds often have roman numerals in them beca. Well learn how to name ionic compounds that have transition metals in them.
For a two element ionic compound the naming is simple. The first part of the name is the name of the metal element. The second part is the name of the nonmetal element with the suffix -ide.
Naming compounds have never been so simple. With my strategy and step by step examples you will be naming compounds like a pro in no time.