While carboxylic acid has the -COOH group the hydrogen is replaced by a hydrocarbon in an ester. HR 3 N HR 2 N HRN ROH.
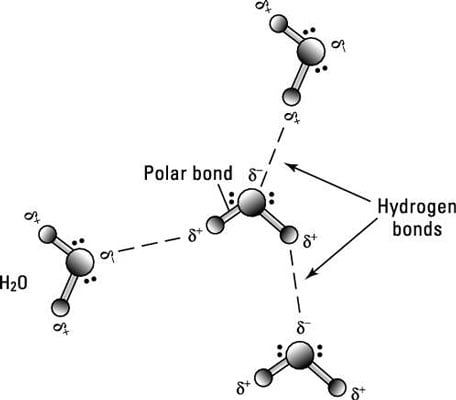
One of the slightly positive hydrogen atoms in a water molecule can be sufficiently attracted to one of the lone pairs on one of the oxygen atoms in an ester for a hydrogen bond to be formed.
Hydrogen bonding for dummies. When studying environmental science one type of atomic bond you need to be familiar with is the hydrogen bond. A hydrogen bond results when some of the atoms in a covalently bonded molecule pull the shared electrons to one side of the molecule creating an electrical imbalance in the molecule. Remember that electrons have a negative electric charge.
In this video we discuss hydrogen bonds. We cover how do hydrogen bonds form the different elements that take part in hydrogen bonds and why doesnt oil a. Hydrogen bonding reduces extreme temperature shifts near large bodies of water.
Hydrogen bonding allows animals to cool themselves using perspiration because such a large amount of heat is needed to. Hydrogen bonding keeps water in its liquid state over a wider temperature range than for any. MOLECULAR HYDROGEN Molecular hydrogen gas or H 2 g is the primary form in which hydrogen is found.
In other words two hydrogen atoms H are covalently bonded a type of chemical bond together as H-H. Because there are two hydrogen atoms we call this diatomic hydrogen di meaning two. Hydrogen bonding interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons.
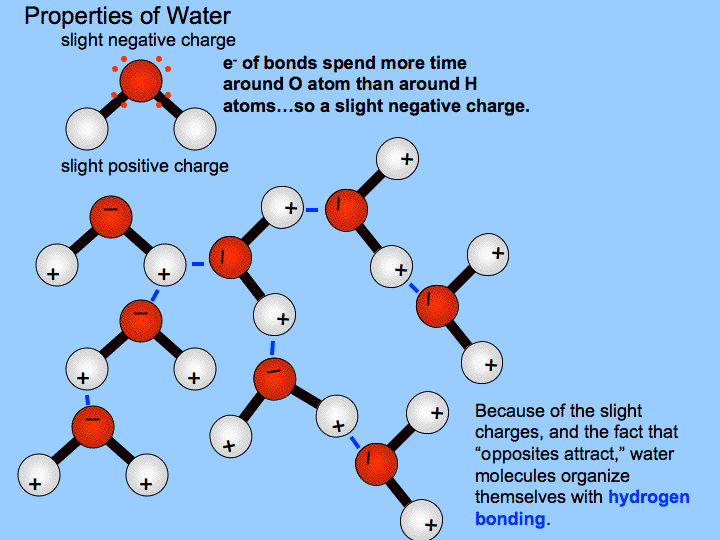
Such a bond is weaker than an ionic bond or covalent bond but stronger than van der Waals forces. Hydrogen bonds can exist between atoms in different molecules or in the same molecule. A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom such as oxygen or nitrogen.
This is not a sharing of electrons as in a. In chemistry a hydrogen bondis a type of attractive intermolecular forcethat exists between two partialelectric chargesof opposite polarity. Although stronger than most other intermolecular forces the hydrogen bond is much weaker than both the ionic bondand the covalent bond.
A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. All of the electron pairsshared and unsharedrepel each other.
Hydrogen bonds are ubiquitous in the body and vary greatly in strength. This introduction to medicinal chemistry is focussed on the both the strength and distance of these bonds and how they influence these reactions. Drug-target hydrogen bond strengths are typically within the range of 16 to 60 kJ mol-1.
Drug-target hydrogen bond distances are typically within the range of 15-22 Å. Often enhanced by ionic interactions. HR 3 N HR 2 N HRN ROH.
Covalent Bonding In covalent bonding electrons are shared between atoms rather than donated in order for the atoms of both elements to gain full outer shells. Electrons are always shared in pairs. An example of covalent bonding is the molecule of carbon dioxide.
In this example carbon has 4 of 8 electrons in its outer shell and oxygen. There are four electron groups around nitrogen three bonding pairs and one lone pair. Repulsions are minimized by directing each hydrogen atom and the lone pair to the corners of a tetrahedron.
With three bonding pairs and one lone pair the structure is designated as AX 3 E. A hydrogen bond is a weak chemical bond that occurs between hydrogen atoms and more electronegative atoms like oxygen nitrogen and fluorine. The participating atoms can be located on the same molecule adjacent nucleotides or on different molecules adjacent nucleotides on different DNA strands.
As you would expect the strength of intermolecular hydrogen bonding and dipole-dipole interactions is reflected in higher boiling points. Just look at the trend for hexane nonpolar London dispersion interactions only 3-hexanone dipole-dipole interactions and 3-hexanol hydrogen bonding. Hydrogen bonding refers to the formation of Hydrogen bonds which are a special class of attractive intermolecular forces that arise due to the dipole-dipole interaction between a hydrogen atom that is bonded to a highly electronegative atom and another highly electronegative atom which lies in the vicinity of the hydrogen atom.
Esters are derived from carboxylic acids and usually alcohol. While carboxylic acid has the -COOH group the hydrogen is replaced by a hydrocarbon in an ester. The chemical formula of an ester takes the form RCO 2 R where R is the hydrocarbon parts of the carboxylic acid and R is the alcohol.
A chemical bond in which a hydrogen atom that is already bonded to an atom in a molecule forms a second bond with another atom either in the same molecule or in a different one. The second atom is usually of a type that strongly attracts electrons such as nitrogen or oxygen. The American Heritage Student Science Dictionary Second Edition.
One of the slightly positive hydrogen atoms in a water molecule can be sufficiently attracted to one of the lone pairs on one of the oxygen atoms in an ester for a hydrogen bond to be formed. There will also of course be dispersion forces and dipole-dipole attractions between the ester and the water molecules.