The physical and chemical properties of pure substances are non-changing if it is on its own without disturbing. A pure substance has constant physical and chemical properties while mixtures have varying physical and chemical properties ie boiling point and melting point.
A mixture can be separated into two or more pure substances.
Is a mixture a pure substance. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together Distinguishing between pure substances and. A pure element or compound contains only one substance with no other substances mixed in. Impure materials may be mixtures of elements mixtures of compounds or mixtures of elements and compounds.
A Pure Substance is matter which cannot be separated into its basic components by using a physical or a chemical process. The physical and chemical properties of pure substances are non-changing if it is on its own without disturbing. A Mixture is made up of a combination of two or more substances that are not united using a chemical reaction.
A pure substance is in the purest form and has no impurities in it while mixture has impurities or is made up of two or more than substances. Pure Substances have sharp melting and boiling point and have standard melting or boiling point contrary to this boiling and melting points of mixtures varies by the proportion of constituents. Water is an example of a pure substance on the other hand salt mixed in water is an example of a mixture.
It cannot be broken down or separated into new products. It can be separated using different separation methods. Constant physical and chemical properties.
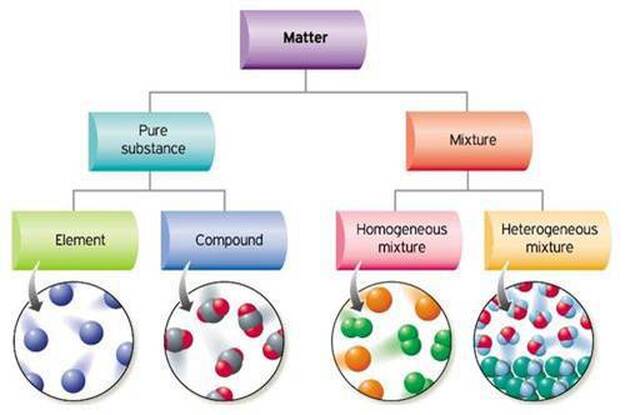
Mixtures have varying physical and chemical properties. Pure substances are made up of a single element. Mixtures are physical combinations of pure substances that have no definite or constant composition the composition of a mixture varies according to who prepares the mixture.
A pure element or compound contains only one substance with no other substances mixed in. Impure materials may be mixtures of elements mixtures of compounds or mixtures of elements and compounds. Mixtures are substances that are composed of two or more different types of particles.
Cement is a mixture because it is composed of different types of compound particles. Each of the components of concrete by themselves would be pure substances. Click to see full answer.
Pure substances cannot be separated into any other kinds of matter while a mixture is a combination of two or more pure substances. A pure substance has constant physical and chemical properties while mixtures have varying physical and chemical properties ie boiling point and melting point. A pure substance is matter that contains only a single substance.
A pure substance cannot break down into a simpler substance. A mixture is matter that is made up of two or more pure substances mixed together. Chocolate chip cookie dough is a mixture of flour sugar eggs salt and cocoa.
A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The components of a mixture can usually be. Isolation purification characterization and identificationedit Often a pure substance needs to be isolated from a mixture for example from a natural sourcewhere a sample often contains numerous chemical substances or after a chemical reactionwhich often give mixtures of chemical substances. In chemistry a pure substance consists of only one type of atom molecule or compound.
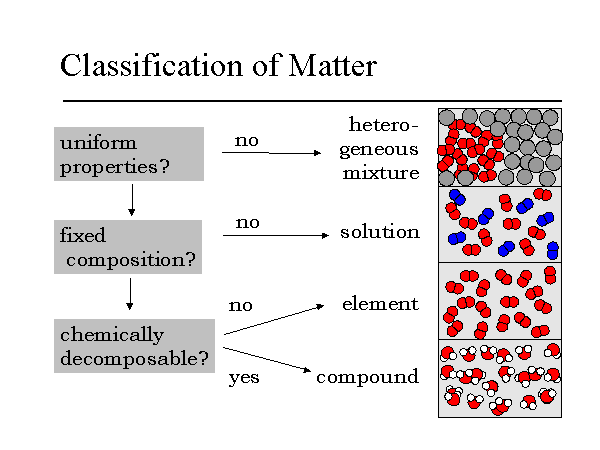
The measure of whether a substance is pure or not is known as purity a pure element or compound contains only one substance with no other substances mixed in. A pure substance consists only of one element or one compound a mixture consists of two or more different substances not chemically joined together The substances in a mixture can be elements or. Chemical and physical properties are constant.
Chemical and physical properties may vary. Individual properties of components are retained. Pure substances can be categorised as gas liquid and solid.
Mixtures are categorised as homogeneous and heterogeneous. Examples include pure water H 2 gas gold. Air is made up of a combination of elements which is a mixture.
Air cannot be pure because it isnt a single substance that can be measured in terms of purity. The components could be called pure but not as a whole. A pure substance is made up solely of that substance and cant be separated into any other substances.
A mixture can be separated into two or more pure substances. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Mixtures are physical combinations of two or more elements andor compounds.
Mixtures can be classified as homogeneous or heterogeneous.