Oxygen can be either a product or a reactant depending on the situation. When one reactant is in excess.

60 g of magnesium reacts with 40 g oxygen to produce magnesium oxide MgO.
Is oxygen a reactant. Oxygen can be either a product or a reactant depending on the situation. As a reactant it undergoes a chemical reaction with something else to make a product. And it could end up as a product of.
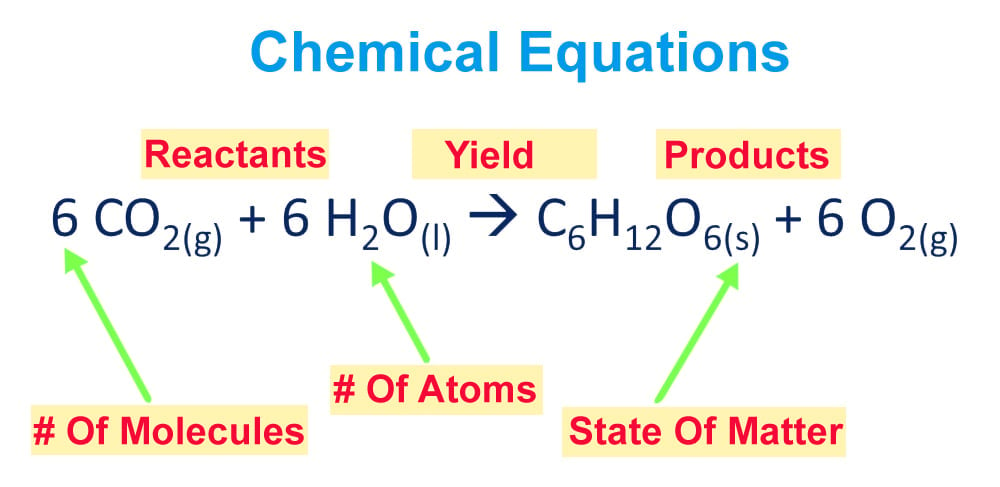
Oxygen and glucose are both reactants in the process of cellular respiration. The main product of cellular respiration is ATP. Waste products include carbon dioxide and water.
What is the formula for cellular respiration. C 6 H 12 O 6 6 O 2 – 6 CO 2 6 H 2 O ATP is the complete balanced chemical formula for cellular respiration. H 2 hydrogen gas and O 2 oxygen gas are reactants in the reaction that forms liquid water.
2 H 2 g O 2 g 2 H 2 Ol. Notice mass is conserved in this equation. The reactant that is all used up is called the limiting reactant.
60 g of magnesium reacts with 40 g oxygen to produce magnesium oxide MgO. Deduce the balanced equation for the reaction. Oxidation is the loss of electrons from a substance.
It is also the gain of oxygen by a substance. For example magnesium is oxidised when it reacts with oxygen to form magnesium oxide. Since the amount of product produced by oxygen is less than that produced by ammonia oxygen is the limiting reactant and ammonia is in excess.
Final words As we can see the limiting reagent or limiting reactant in a reaction is the reactant that gets completely exhausted and thus prevents the reaction from continuing forward. The other reactant has nothing left to react with so some of it is left over. 608 g of magnesium reacts with 400 g oxygen to produce magnesium oxide MgO.
Deduce the balanced equation for. A good way to ensure that one reactant fully reacts is to use an excess of the other reactant. This is financially efficient when one of the reactants is very cheap.
When one reactant is in excess. Solution for In the following reaction oxygen is the excess reactant. SiCl4 O2 SiO2 Cl2 The table shows an experimental record for the above reaction.
The substances which are present at the starting of a chemical reaction are known as reactants. In the burning of natural gas for example methane CH4 and oxygen O2 are the reactants in the chemical reaction. Water as a reactant in hydrolysis.
The simplest chemical reaction in which a water molecule is a reactant is known as hydrolysis reaction. Carbon reacts with oxygen to produce carbon dioxide. Cs O2g CO2g Calculate the maximum mass of carbon dioxide that can be made from 60 g of carbon and an excess of oxygen.
Oxygen produces fewer moles of water and therefore oxygen is the limiting reactant. Oxygen will be completely consumed once 4 moles of H 2 O have been produced. The stoichiometry between hydrogen and oxygen being 21 four moles of hydrogen are needed to react with two moles of oxygen.
Surface lattice oxygen is the key active site on the metal oxide but its role and activation mechanism in the photocatalytic VOC mineralization are still unclear. In this work we have demonstrated that Sr2Sb2O7 exhibits an excellent photocatalytic activity and stability compared to TiO2 P25 in gaseous toluene mineralization because the lattice oxygen on Sr2Sb2O7 can be activated efficiently. A substance that is part of a chemical reaction 2.
A substance that is part of a chemical. Thus 046 moles of CS2 reacts with 3 046 moles of O2 which is 138 moles of O2 Thus oxygen is the limiting reactant with 188 - 138 049605 mole remaining Or 8g of oxygen 589g of SO2 is produced. Here is the reactant.
On the other hand both and are the products. Thus we can conclude that there are three oxygen atoms in the reactant and there are three oxygen atoms in the product. Updated November 04 2019 An oxidant is a reactant that oxidizes or removes electrons from other reactants during a redox reaction.
An oxidant may also be called an oxidizer or oxidizing agent. When the oxidant includes oxygen it may be called an oxygenation reagent or oxygen-atom transfer OT agent.