
Sand is added to water Classification. A few of them include gold copper oxygen chlorine diamond etc.
Sand is a heterogeneous mixture because it is a granular substance composed of small particles of rocks or minerals.
Is sand a pure substance. Actually sand is not a pure substance. There is no chemical formula for sand as it is a mixture. However the water if it is distilled is a pure substance with the chemical formula of H2O.
I would say no because in my oppion a pure substance is somthing that is just 1 repeated molicule and sand has salt and rocks and all sorts of other small stuff. Because substance could contain. Sand is a heterogeneous mixture because it is a granular substance composed of small particles of rocks or minerals.
A heterogeneous mixture contains components that are not uniform throughout the mixture. For example sand can contain different grain sizes and small colored particles. Some other examples of heterogeneous mixtures are blood gravel and the combination of oil and vinegar.
Primarily of SiO2 but. With other minerals. Or eroded rock material.
Although water is a pure substance if you put sand into a glass of water it would turn into a mixture. Each of the components of a mixture can be separated from one another. You can always separate the sand from water by filtering it.
Pure substances have a uniform chemical composition. Examples include water liquid diamond solid and oxygen gas. In chemistry a pure substance is a material with a constant composition.
Common Definition of a Pure Substance. To a non-chemist a pure substance is anything composed of a single type of material. In other words it is free of contaminants.
So in addition to elements compounds and alloys a pure substance might include honey even though it consists of many different types of molecules. For the best answers search on this site httpsshorturlimavuaK. A b and d are pure substances c and e are mixtures These are the ideal answers in an ideal world.
In our real world water is never pure water it contains many dissolved minerals so it is a type of mixture called a solution. The melting point and the boiling point of the mixtures depend on the presence of the different compounds. Examples of pure substances can be H2 gas oxygen gas gold pure water etc.
Examples of the mixture can be- sugar sand alcohol ammonia etc. Steel combination of many metals Mixture. Chocolate syrup is added to milk and stirred Classification.
Homogeneous mixture solution Copper metal used to make wires Classification. Sand is added to water Classification. Homogeneous solution Tap water.
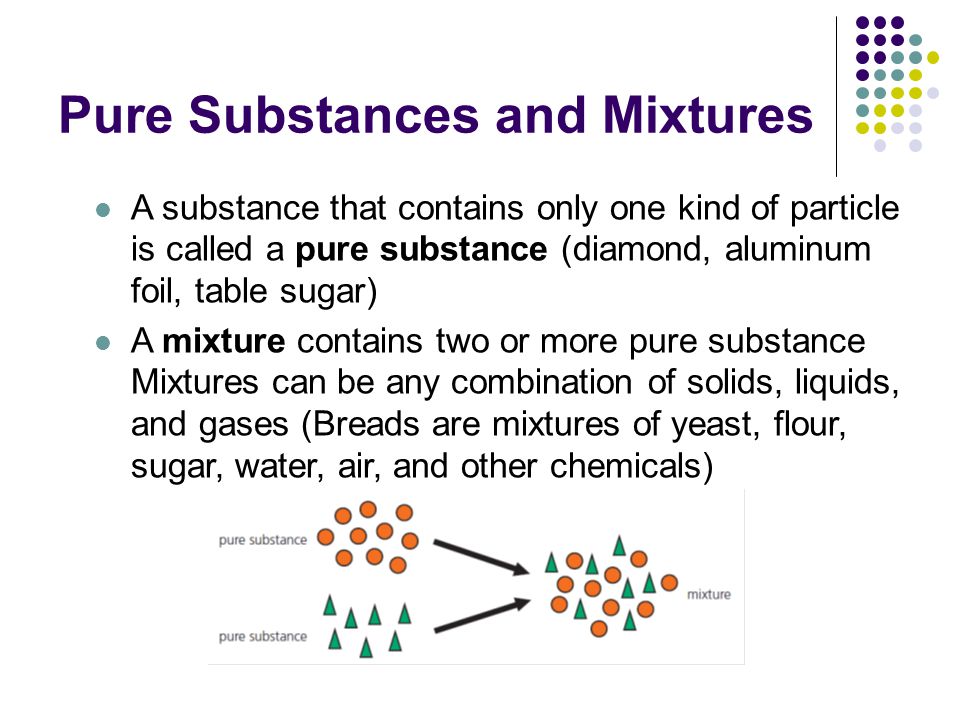
Chemists can classify matter as solid liquid or gas. But there are other ways to classify matter as well such as pure substances and mixtures. Classification is one of the basic processes in science.
All matter can be classified as either a pure substance or a mixture. The classification of matter. Pure substances A.
Pure substances and mixtures The meaning of pure. The word pure is used in chemistry in a different way from its everyday meaning. For example shops sell cartons labelled as pure orange juice.
A pure substance usually participates in a chemical reaction to form predictable products. Examples of Pure Substances. All elements are mostly pure substances.
A few of them include gold copper oxygen chlorine diamond etc. Compounds such as water salt or crystals baking soda amongst others are also grouped as pure substances. For each of the following substances state whether it is a pure substance or a mixture.
If it is a mixture is it homogeneous or heterogeneous. If it is a pure substance is it an element or a compound. Blood which is made up from plasma and cells Argon.
Silicon dioxide textSiO_2 Sand and stones. When we speak of a pure substance we are speaking of something that contains only one kind of matterThis can either be one single element or one single compound but every sample of this substance that you examine must contain exactly the same thing with a fixed definite set of properties. Pure substances have a specific boiling point and a specific melting point.
Concerning electrical conductivity the copper used in electrical wiring must be pure. A substance such as pure liquid water is a very poor conductor of electricity due to the lack of electrolytes that assist in the conduction of electricity. Is sand considered a pure substance.
What is considered to be a pure substance and what are some. Sand is a mixture of various substances. Clay is a type of fine-grained natural soil material containing clay minerals.
Clays develop plasticity when wet due to a molecular film of water surrounding the clay particles but become hard brittle and nonplastic upon drying or firing. Most pure clay minerals are white or light-coloured but natural clays show a variety of colours from impurities such as a reddish or brownish colour. Compound pure substance mixture and an element.
Matter is anything that occupies space and has mass. A matter can be distinguished from another matter based on its physical and.