If so you have a pure substance. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas.

The matter exists in three fundamental phases.
Is water a mixture or pure substance. Water is a pure substance. This is because it has the same material all over and is made of one kind of substance throughout. Moreover water cannot be separated into other materials.
Water is a compound that contains two different elements. When salt is added into water it becomes a mixture. Water is a mixture because it is made up of molecules that are composed of two atoms of hydrogen and one atom of oxygen.
An atom is the smallest particle of a pure substance element that has all. Diamond only contains the element carbon. Air is a mixture of oxygen carbon dioxide nitrogen and other trace gases.
Water only contains the compound water. Water is a pure substance if you put sand into a glass of water it would turn into a mixture. More facts and examples of pure substances and mixtures.
A compound is composed of two or more elements in a specific ratio. For example water is a compound made up of two elements hydrogen H and oxygen O. These elements are combined in a very specific way in a ratio of two hydrogen atoms to one oxygen atom known as.
Distilled deionized pure water- When water does not have any ions dissolved or any other substance dispersed it is a pure substance in liquid form. However water is often found naturally as a homogeneous mixture with various other substances dissolved in it. Mixtures are the substances that are made up of the combination of two or more substances and these substances can be separated by the physical and the chemical processes.
Some of the examples of pure substances can be pure water gold platinum etc whereas some of the examples of the mixtures can be carbon dioxide gas sugar alcohol etc. Water H2O is a pure substance a compound made of hydrogen and oxygen. Although water is the most abundant substance on earth it is rarely found naturally in its pure form.
Most of the time pure water has to be created. Pure water is called distilled water or deionized water. Pure substances cannot be separated into other substances.
Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. If so you have a pure substance. If not you have a mixture.
Pure Substances have sharp melting and boiling point and have standard melting or boiling point contrary to this boiling and melting points of mixtures varies by the proportion of constituents. Water is an example of a pure substance on the other hand salt mixed in water is an example of a mixture. In science a pure substance contains only one element or compound.
Mineral water is mostly water but there are other substances mixed with it. These are the ingredients that you see listed on the. A pure substance is something that is made up of only one type of particle.
A pure substance cannot be physically separated into other substances because all of the particles are the same. Pure substances have fixed physical properties such as melting and boiling point. Examples of pure substances include oxygen water and iron.
Elements like sodium are pure substances because their molecular structures consist of just one type of atom. Similarly pure water that has nothing but two hydrogen atoms and one oxygen atom is a pure substance. Things like lemonade which are a mix of water sugar and lemon juice are mixtures rather than pure substances.
The matter exists in three fundamental phases. Gas liquid and solid. Apart from these the matter can be either a pure substance or mixture.
A pure substance is made up of only one type of particle and has a fixed structure. A mixture on the other hand is the physical combination of two or more substances. Pure Substances or Mixture.
Copper metal used to make wires Pure Substance. Sand is added to water. Helium gas used to inflate a balloon Pure Substance.
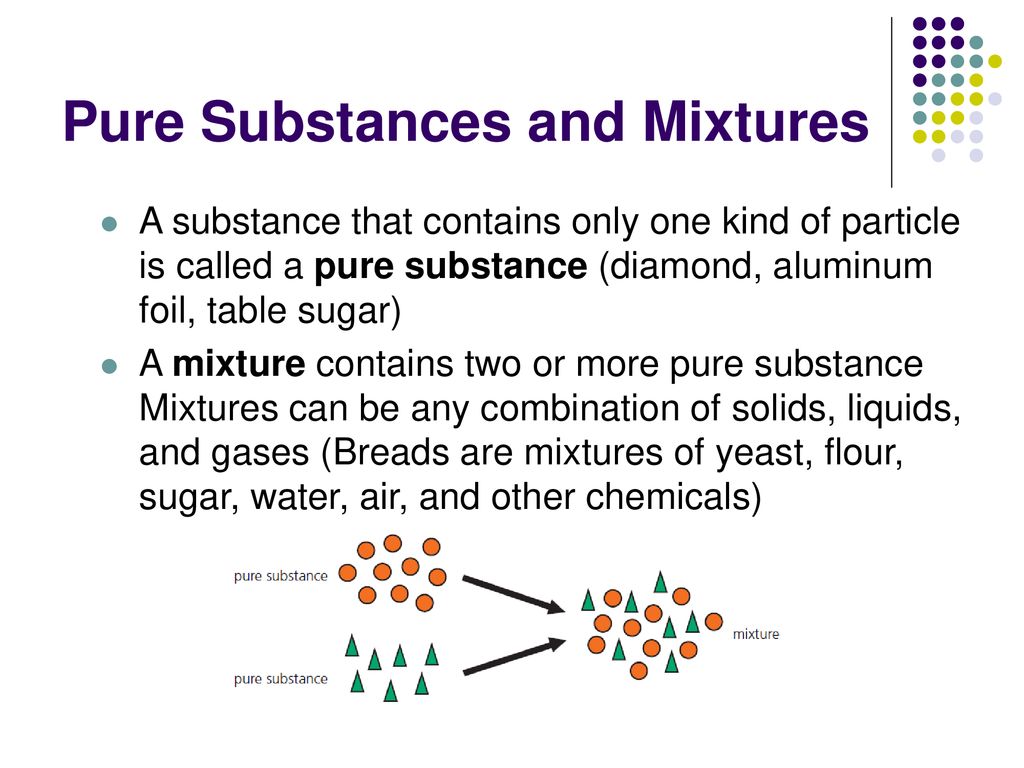
Mixture contains two or more pure substance Mixtures can be any combination of solids liquids and gases Breads are mixtures of yeast flour sugar water air and other chemicals. Heterogeneous mixtures are not uniform throughout however this mixture is uniform so it is not a heterogeneous mixture. This liquid is not a pure substance because two compounds salt and the.