
Can a metalloid have properties of metals and nonmetals. Metals non-metals and metalloids Moving from left to right across a period the elements become less metallic.
Lets start by looking at the periodic table and working out where we can find metals nonmetals and metalloids.
Metal nonmetal and metalloid. Metals non-metals and metalloids Moving from left to right across a period the elements become less metallic. This is related to the increase in the number of electrons in the outer shell of. The metalloids or semimetals have properties that are somewhat of a cross between metals and nonmetals.
Metalloids tend to be economically important because of their unique conductivity properties they only partially conduct electricity which make them valuable in the semiconductor and computer chip industry. Metalloids are metallic-looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides. Typical nonmetals have a dull coloured or colourless appearance.
Are brittle when solid. Are poor conductors of heat and electricity. And have acidic oxides.
Metals nonmetals and metalloids are elements that are found in the earth. Most of these elements are used in various applications. The main difference between metals nonmetals and metalloids is that metals show the highest degree of metallic behavior and nonmetals do not show metallic behavior whereas metalloids show some degree of metallic behavior.
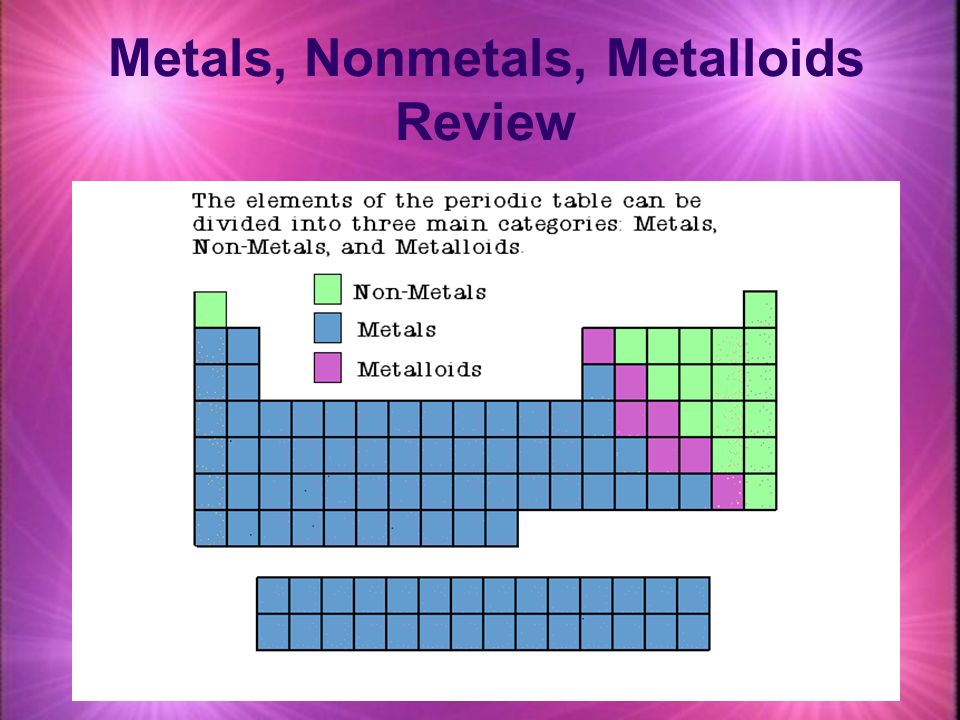
The metals are to the left of the line except for hydrogen which is a nonmetal the nonmetals are to the right of the line and the elements immediately adjacent to the line are the metalloids. When elements combine to form compounds there are two major types of bonding that can result. The main difference between metals non-metals and metalloids are that metals are elements that are hard malleable fusible shiny ductile and good conductors.
Non-metals do not have properties present in metals whereas metalloids are elements that have intermediate properties of both metals and non-metals. Nonmetals have a high electronegativity. Metalloids have an intermediate value of electronegativity.
Malleability and Ductility. Metals are malleable and ductile in nature. Nonmetals are neither malleable nor ductile in nature.
Just like nonmetals metalloids are neither ductile nor malleable in nature. Block In The Periodic Table. Metalloids have properties intermediate between the metals and nonmetals.
Metalloids are useful in the semiconductor industry. Metalloids are all solid at room temperature. They can form alloys with other metals.
Metals are the elements which exhibit the highest degree of metallic behavior is known as metals on the contrary Non-metals are such elements which do not possess any metallic behavior and Metalloids are those elements that possess some of the properties like metal while some like non-metal. In this video we will look at what we mean by the terms metal nonmetal and metalloid as well as exploring the properties of each of these. Lets start by looking at the periodic table and working out where we can find metals nonmetals and metalloids.
Here we have a rough outline of our periodic table. Generally speaking metals can be. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals.
The metalloids separate the metals and nonmetals on a periodic table. Also many periodic tables have a stair-step line on the table identifying the element groups. The line begins at boron B and extends down to polonium Po.
Identify each of the following elements as a metal a nonmetal or a metalloid. A shiny element d. An element that is a gas at room temperature e.
Located in Group 8 mathrmA18 f. VINotebook Metals Non-metals MetalloidsMpptx - Metals Nonmetals and Presented by Kesler Science Metalloids Vers 072020 u00a9 Kesler Science LLC Reflect. Somewhere between metals and nonmetals lie metalloids or semiconductors Let us identify the given elements as metal nonmetal or metalloid.
Sulfur S is found on the right side of the. In chemistry a nonmetal or non-metal is a chemical element that mostly lacks the characteristics of a metalPhysically a nonmetal tends to have a relatively low melting point boiling point and densityA nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivityChemically nonmetals tend to have relatively high ionization energy. Where at the metalloids located on the Periodic Table.
Where is between metals and metalliods. Can a metalloid have properties of metals and nonmetals. SOLIDS LIQUIDS or GASSES.
Pollution index and health risk index are tools used to assess elemental contamination in the environment and food. A total of 6 metals Al Ba Ca Cu Fe and Zn one nonmetal Se and one metalloid As were quantified in the samples of the canned tuna and canned sardines. For elements as Al Cu Fe and Se PI 1.