
It is the ratio of the mass of solute to the mass of solution multiplied by 100 to calculate mass percent. Find the molar mass of each element using the periodic table of elements.
Another way to specify an amount is percentage composition by mass or mass percentage mm.
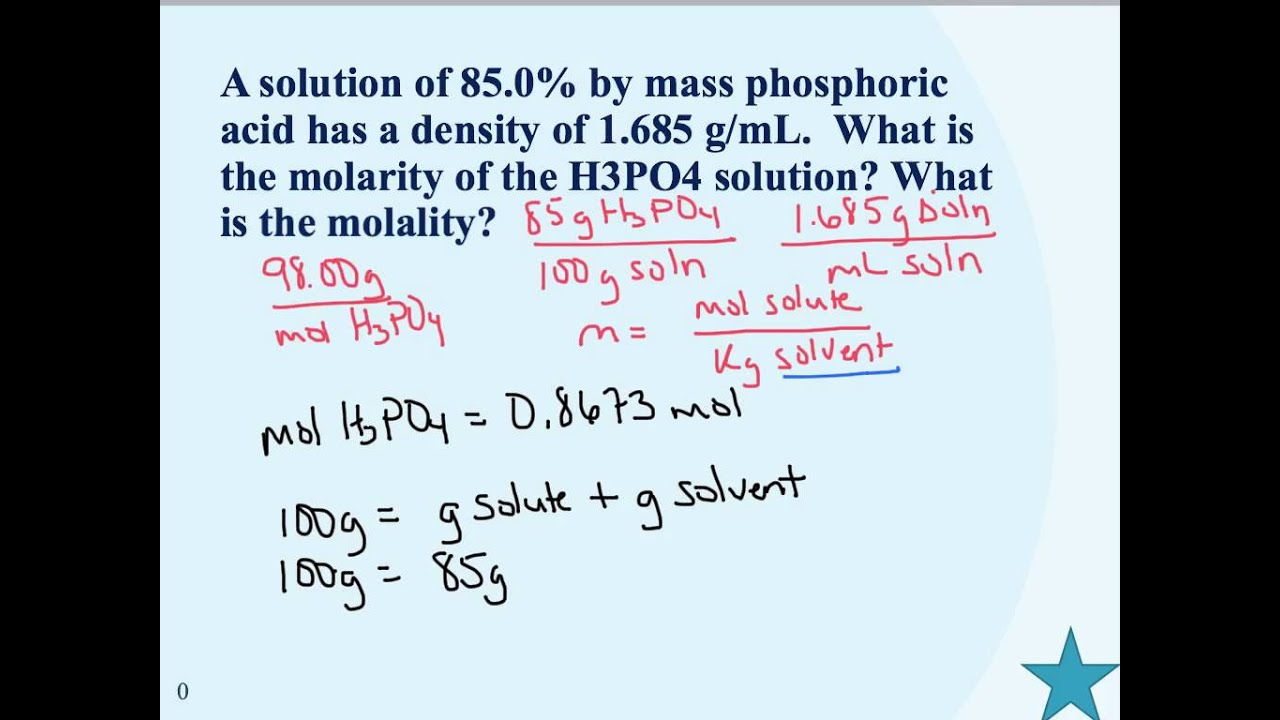
Molarity from mass percent. How to convert molarity to percentage concentration. Heres the equation we use to convert the percentage concentration to molarity. Molarity Percentage concentration Density Molar mass 100 The units required for this calculation are.
Molarity - moldm³ M molL. Percentage concentration - Density - gL gdm³. The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar concentration and volume.
To dilute a solution of known molarity please use the Solution Dilution Calculator. To dilute a solution of concentrated acid or base of known ww strength please use the Acid Base Molarity Calculator. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Next calculate the mass-volume percent solution. Note that the convention in molarity is to divide moles by liters but the convention in mass percent is to divide grams by milliliters. If you prefer to think only in terms of liters not milliliters then simply consider mass percent as kilograms divided by liters.
Mathematical manipulation of molality is the same as with molarity. Another way to specify an amount is percentage composition by mass or mass percentage mm. It is defined as follows.
The formula of the mass per cent is given as. Mass percent fracmass of solutemass of solution 100. Let us now look at some solved examples of molarity to know in detail about what is molarity in chemistry.
First define the percentage solution. 085 wv solution 085 g100 mL. Since molarity is defined in terms of gL convert 085 g100 mL to gL.
Once the number of grams of solute per liter is known the molarity can be calculated. X 85 gGMW x 10. GMW Na 2299 Cl 3545 5844 X 855844.
Mass of X in g X in molL molar mass of X in gmolvolume in L of X Do the same for compound X-1 X-2 and however many compounds you have unless you know the mass of the total mixture. Then add all the X masses together. Weight percent Mass of Xmass of X mass of X-1 Mass of X-n 100.
In this video we look at how to calculate the molarity of a solution when you are given the mass percent and density of that solution. Not too difficult once. Calculate the molality mass percent and mole fraction of nitric acid in the solution.
1 Assume 1000 L of the solution is present. ML 1432 g. 2 Determine the mass percent just the nitric acid.
1600 mol 630119 gmol 100819 g 100819 g 1432 g 7040. The molarity calculator tool provides lab-ready directions describing how to prepare an acid or base solution of specified Molarity M or Normality N from a concentrated acid or base solution. To prepare a solution from a solid reagent please use the Mass Molarity Calculator.
Use the density of the solution to get the total volume of the solution. Then use the weight percent of solute to determine the amount of substance of the solute. Use the amount of substance of the solute divided by the volume to get molarity.
Molarity is relevant to our concept of chemistry and kinetics of reactions. 0007 Statement of problem0043 Assume mass 100 g0113 Convert mass percents to actual masses0147 Calculate molar mass of KBr0245 Convert mass. Find the molar mass of each element using the periodic table of elements.
Make sure that you count the atoms for each element and calculate the molar mass of each of the atoms. Molar mass of K 391 g. Molar mass of Mn 549 g.
Molar mass of O 160 g The solute contains 4 O atoms so count the 16g 4 times. The molar mass of atoms of an element is given by the relative atomic mass of the element multiplied by the molar mass constant M u 1000 000 10 3 kgmol 1000000 gmol. For normal samples from earth with typical isotope composition the atomic weight can be approximated by the standard atomic weight or the conventional atomic weight.
Molality is also known as molal concentration. It is a measure of solute concentration in a solution. The solution is composed of two components.
There are many different ways to express the concentration of solutions like molarity molality normality formality volume percentage weight percentage and part per million. It is the ratio of the mass of solute to the mass of solution multiplied by 100 to calculate mass percent. It is also known as weight percent and is represented by ww.
You may have seen this symbol on the back of medicines and tablets. It is one of the most commonly used units of representing concentration. The molar mass of a compound sometimes to referred to as molecular weight is the cumulative atomic weight of all the atomselements in the compound.
For example 1 mole of water H 2 O has a molar mass of roughly 180 grams. Finding Mass Percentage and using it to determine an Empirical Formula.