
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. Further the atom is made up of two parts or regions the central part called the nucleus and atomic orbits.
Therefore it is neutral.
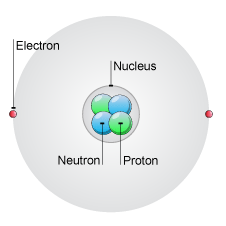
Nucleus of an atom contains. The compact positively-charged nucleus of an atom contains two main types of subatomic particles. Orbiting the atoms nucleus are negatively-charged subatomic particles called electrons. The mass of an atom is concentrated in the nucleus.
The nucleus of an atom consists of neutrons and protons which in turn are the manifestation of more elementary particles called quarks that are held in association by the nuclear strong force in certain stable combinations of hadrons called baryons. The protons and neutrons form a very small dense core known as the nucleus. Therefore most of the mass of an atom is contained in its nucleus.
Electrons move in orbits around the nucleus. The diameter of the atom is about 100 000 times bigger than the diameter of the nucleus. This means that there is a lot of empty space within an atom.
The nucleus plural nuclei is defined as the dense central part of an atom consisting of two subatomic particles namely protons and neutrons. A nucleus accounts for more than 999 of an atoms mass but is 100000 times smaller than it in size. They are thus the densest part of an atom.
The word nucleus means kernel of a nut. The nucleus of an atom contains A. Protons neutrons and electrons.
Isotopes of a given element have A. The same number of protons but differ in atomic mass. The same atomic mass but different number of protons.
Different number of electrons. Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons.
Only the most common variety of hydrogen has no neutrons. More than 9994 of an atoms mass is in the nucleus. Thomson proposed that the nucleus of an atom contains only nucleons.
Ii A neutron is formed by an electron and a proton combining together. Therefore it is neutral. Iii The mass of an electron is about 12000 times that of proton.
A helium nucleus was presumed to be composed of four protons plus two nuclear electrons electrons bound inside the nucleus to cancel two of the charges. At the other end of the periodic table a nucleus of gold with a mass 197 times that of hydrogen was thought to contain 118 nuclear electrons in the nucleus to give it a residual charge of 79 consistent with its atomic number. Further the atom is made up of two parts or regions the central part called the nucleus and atomic orbits.
The particles found in the atom are electrons protons and. Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells. The number of subatomic particles in an atom can be calculated from the atoms atomic number and mass.
A JJThomson proposed that the nucleus of an atom contains only nucleons. B A neutron is formed by an electron and a proton combining together. Therefore it is neutral.
The nucleus that dense central core of the atom contains both protons and neutrons. Electrons are outside the nucleus in energy levels. Protons have a positive charge neutrons have no charge and electrons have a negative charge.
A neutral atom contains equal numbers of protons and electrons. A nucleus of a carbon12 isotope contains six protons and six neutrons while a nitrogen14 nucleus comprises seven protons and seven neutrons. A graduate student performs a nuclear physics experiment in which she bombards nitrogen14 nuclei with very high speed carbon12 nuclei emerging from a particle accelerator.
The nucleus is like the brain of the atom. It consists of protons and neutrons. Protons have a positive charge and neutrons have a neutral charge no charge.
The nucleus sits in the middle of the. Solution for The nucleus of an atom is composed of. If the nucleus of an atom containing 12 protons how many electrons are there in a neutral atom.
Which statement describes the structure of an atom. 1 The nucleus contains positively charged electrons. 2 The nucleus contains negatively charged protons.
3 The nucleus has a positive charge and is surrounded by negatively charged electrons. 4 The nucleus has a negative charge and is surrounded by positively charged electrons. The nuclei of all atoms contain subatomic particles called protons.
The nuclei of most atoms also contain neutrons. The nucleus of the simplest atom - hydrogen - contains only one proton and no. Numerically the nucleus of an atom possesses almost nearly 10-14 times the volume of the atom yet contains 9999 of the atomic mass.
The nucleus of an atom is little to the point that if you extended an atom to occupy a room the nucleus of an atom would at present be no bigger than a pinhead.