All the metal in a compound generally possesses a positive oxidation state. This is an electrically neutral compound and so the sum of the oxidation states of the hydrogen and oxygen must be zero.

Possible oxidation states are 1-1.
Oxidation number of hydrogen. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H. Electron Configuration and Oxidation States of Hydrogen.
Electron configuration of Hydrogen is 1s1. Possible oxidation states are 1-1. To find the correct oxidations state of H2 Hydrogen gas and each element in the molecule we use a few rules and some simple mathFirst since the H2 mole.
Since Group 1 metals always have an oxidation state of 1 in their compounds it follows that the hydrogen must have an oxidation state of -1 1 -1 0. Peroxides include hydrogen peroxide H 2 O 2. This is an electrically neutral compound and so the sum of the oxidation states of the hydrogen and oxygen must be zero.
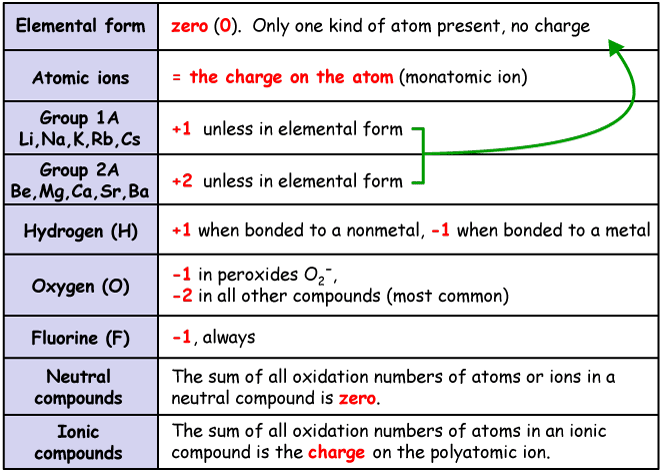
The oxidation number of hydrogen is 1 when it is combined with a nonmetal as in CH 4 NH 3 H 2 O and HCl. The oxidation number of hydrogen is -1 when it is combined with a metal as in. LiH NaH CaH 2 and LiAlH 4.
Hydrogen has an oxidation number of 1 when combined with non-metals but it has an oxidation number of -1 when combined with metals. The algebraic sum of the oxidation numbers of elements in a compound is zero. The oxidation number for Hydrogen is usually 1 except that in standard state it is 0 and in metallic hydrides such as Li H and Na H it is -1.
Be aware that ammonia NH 3 and AsH 3 are not hydrides since N and as are nonmetallic so H is assigned a value of 1 in both cases. Hydrogen has an oxidation number of 1 in each of H 2 O and H 2 SO 4. Oxidation is loss of electrons gain of oxygen or loss of hydrogen.
Reduction is gain of electrons loss of oxygen or gain or hydrogen. Rusting is an example of oxidation. If the hydrogen is part of a binary metal hydride compound of hydrogen and some metal then the oxidation state of hydrogen is 1.
The oxidation number of fluorine is always 1. Chlorine bromine and iodine usually have an oxidation number of 1 unless theyre in combination with an oxygen or fluorine. The number of electrons that can be gained or lost by the element is called its oxidation state.
The oxidation state of the elements can be positive negative or it can be zero also. Oxidation number is defined as the total number of electrons that are gained or lost by the atom to form a chemical bond. The oxidation number of the compound H₂S is 0.
The sum of the oxidation numbers of the individual elements should add up to the oxidation number of the compound. Oxidation number of H x 2 H ions oxidation number of S 0. Hydrogen has higher electronegativity of 21 than Ca 10.
Hence Ca is e reductantie it provides 2 electrons and the two H atoms are oxidizers taking 1 electron each one on its partially free 1S orbital Thus hydrogen has -1 degree of oxidation. Ca has 2 degree of oxidation. From our oxidation number rules we know that hydrogen typically has an oxidation number of 1 in compounds unless it is bonded to a metal such as in a metal hydride in which case it has an.
The common oxidation number of hydrogen 1 but in alkali metal hydrides like lithium hydride sodium hydride cesium hydride are the examples of the molecules where the oxidation state of hydrogen atom -1. All the metal in a compound generally possesses a positive oxidation state. The hydrogen atom H exhibits an oxidation state of 1.
However when bonded with an element with less electronegativity than it it exhibits an oxidation number of -1. Oxygen has an oxidation of -2 in most of its compounds. However in the case of peroxides the oxidation number corresponding to oxygen is -1.
Oxidation is loss of hydrogen. Reduction is gain of hydrogen. Notice that these are exactly the opposite of the oxygen definitions.
For example ethanol can be oxidised to ethanal. You would need to use an oxidising agent to remove the hydrogen from the ethanol. A commonly used oxidising agent is potassium dichromateVI solution acidified with.
The usual oxidation number of hydrogen is 1. The oxidation number of hydrogen is -1 in compounds containing elements that are less electronegative than hydrogen as in CaH 2. The oxidation number of oxygen in compounds is usually -2.
To find the correct oxidation state of O in H3O the Hydronium ion ion and each element in the ion we use a few rules and some simple mathFirst since t. In our water example hydrogen is assigned an oxidation number of 1 because each individual hydrogen has lost one electron. Oxygen has an oxidation number of 2 because the single oxygen atom has gained a total of two electrons one from each hydrogen.
Here is another molecule involving hydrogen and oxygenhydrogen peroxide H 2 O 2.