
What is an atom. Positively charged ions are called cations.
Na Al3 Ce3 etc.
Positively charged ions are called. An ion is an atom or group of atoms with a positive or negative charge. Ions form when atoms lose or gain electrons to obtain a full outer shell. Metal atoms lose electrons to form positively.
Cations positively-charged ions and anions negatively-charged ions are formed when a metal loses electrons and a nonmetal gains those electrons. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound such as sodium chloride. Positively charged ions are called cations.
Negatively charged ions anions. Ions are formed by the addition of electrons to or the removal of electrons from neutral atoms or molecules or other ions. By combination of ions with other particles.
Or by rupture of a covalent bond between two atoms in such a way that both of the electrons of the bond are left in association with one of the formerly bonded atoms. A positive ion called be called an cation. An atom can become a cation when it either looses an electron or donates its to another atom so that it attains a positive charge.
This can only take place when the atom has to get a stable noble gas electronic configuration otherwise a stable atom say He would not lend its electron. In chemistry an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bondingThe compound is neutral overall but consists of positively charged ions called cations and negatively charged ions called anionsThese can be simple ions such as the sodium Na and chloride Cl in sodium chloride or polyatomic species such as the ammonium. Electrolysis Ionic substances contain charged particles called ions.
For example lead bromide contains positively charged lead ions and negatively charged bromide ions. Electrolysis is the process. Electrons have a negative charge whereas protons have a positive charge.
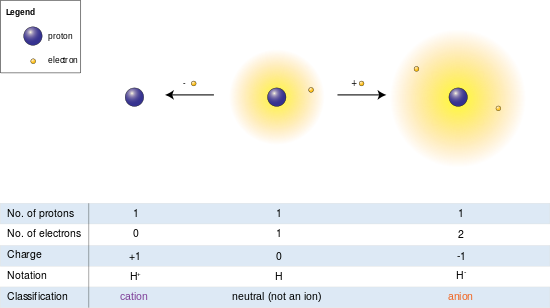
When an atom gains electrons this results in a negative charge. This type of ion is called an anion. When an atom loses electrons this results in a positive charge.
A positively charged ion is called a cation. Lets explore several ion examples of both types. Molecular ions that contain at least one carbon to hydrogen bond are called organic ions.
If the charge in an organic ion is formally centred on a carbon it is termed a carbocation if positively charged or carbanion if negatively charged. Formation Formation of monatomic ions. A positively charged ion is called an anion.
Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. Positively charged ions are called cations. Most metals become cations when they make ionic compounds.
A Positivley charged ion is called an. A substance that gains electrons. Becomes negatively charged while a substance that loses electrons becomes positively charged.
Atoms or molecules that become charged are called ions. An ion with a positive charge is called a cation you can remember the difference between cations and anions negatively charged atoms by noting that cation has a t which looks like a. Cations are caused when an atom in a neutral state loses some of its negatively charged particles called electrons.
Those ions which possess positive charge are called cations. Na Al3 Ce3 etc. Are examples of cations.
When an atom loses its electrons then it gains a positive charge due to less number of electrons than protons in its nucleus. Then this positively charged species is called cation. The compounds which are made up of ions are known as ionic compounds.
In an ionic compound the positively charged ions cations and negatively charged ions anions are held together by the strong electrostatic forces of attraction. The forces which hold the ions together in an ionic compound are known as ionic bonds or electrovalent bonds. Electrons are a form of positive ions and protons are a form of negative ion.
When these two types of ions meet it results in the destruction of atoms in the process of electrons leaving a positively charged atom and becoming a negatively charged atom. Solution for Positively charged ions are called and migrate toward the in an electrical field. Are negatively charged ions which migrate towards the.
When an atom is attracted to another atom because it has an unequal number of electrons and protons the atom is called an ION. If the atom has more electrons than protons it is a negative ion or ANION. If it has more protons than electronsit is a positive ion.
What is an atom.