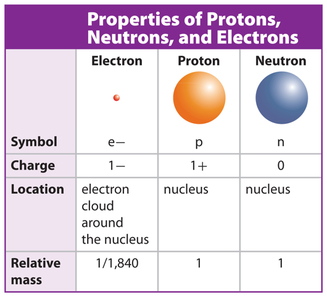
In fact the number of protons in each atom is its atomic number. Protons are found in the nucleus of the atom.
For example a helium atom always has two protons and carbon always has six.
Proton location in atom. In shells around nucleus. The nucleus of an atom is its central body holding particles called protons and neutrons. The number of protons in the nucleus is the atomic number of the element.
For example a helium atom always has two protons and carbon always has six. Protons reside in the nucleus of the atom which might seem strange since they are positively charged and thus repel each other. To form an atomic nucleus however protons that are very close to.
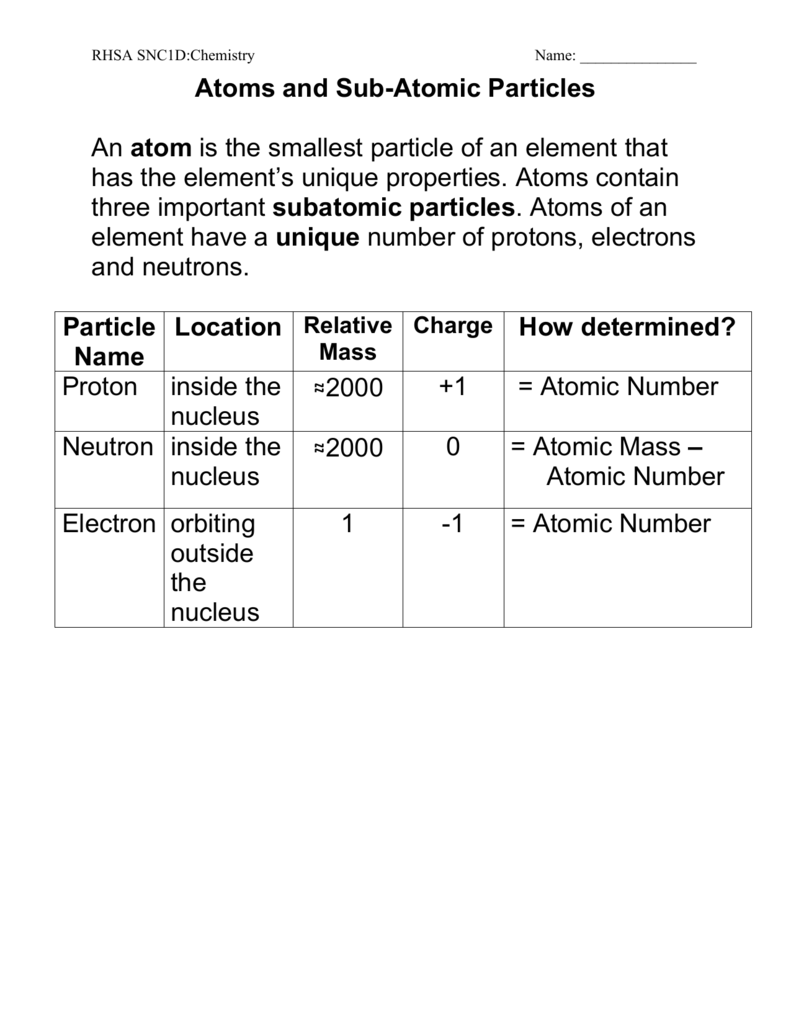
A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny dense region at the center of the atom.
Protons have a positive electrical charge of one 1 and a mass of 1 atomic mass unit amu which is about 167 10 27 kilograms. A proton is a subatomic particle symbol p or p with a positive electric charge of 1e elementary charge and a mass slightly less than that of a neutron. Protons and neutrons each with masses of approximately one atomic mass unit are collectively referred to as nucleons.
One or more protons are present in the nucleus of every atom. They are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element and is referred to as the atomic number.
Protons together with electrically neutral particles called neutrons make up all atomic nuclei except for the hydrogen nucleus which consists of a single proton. Every nucleus of a given chemical element has the same number of protons. This number defines the atomic number of an element and determines the position of the element in the periodic table.
Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Atoms consist of a nucleus containing protons and neutrons surrounded by electrons in shells.
The number of subatomic particles in an atom can be calculated from the atoms atomic number and mass. Proton is another major particle of an atom with a charge of positive polarity. Its a crucial component of an atom that forms the nucleus of an atom with the neutron.
As the nucleus of the atom is at the center thus a proton holding a positive charge is present at the center of the atomic structure. It is denoted by a symbol p and has a charge 1. Within each atom the protons inside the nucleus attract the electrons that are outside the nucleus.
You can experience a similar attractionrepulsion phenomenon with magnets. If you place the north pole of a bar magnet near the south pole of a second bar magnet youll find that the magnets attract each other. Atoms are made of protons neutrons and electrons.
Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. A neutral atom has the same number of protons and electrons charges cancel each other out. An ion has an unequal number of protons and electrons.
Protons make the nucleus or center of an atom. The simplest atoms hydrogen atoms have a nucleus made of just one proton. But most atoms have more than one proton and at least one neutron to go with each proton some have more than one.
Protons are actually made of even smaller invisible particles called quarks. Each proton has three quarks two up quarks and one down quark. Charge Of A Proton.
A proton is a subatomic particle with a positive electric charge. Protons are in every atoms nucleus. In fact the number of protons in each atom is its atomic number.
The proton is now considered a fundamental particle. A proton is a subatomic particle with a positive charge. It is found in the atomic nucleus.
A proton is a subatomic particle found in the nucleus of every atom. The particle has a positive electrical charge equal and opposite to that of the electron. If isolated a single proton would have a mass of only 1673.
10 -27 kilogram just slightly less than the mass of a neutron. Protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. Electrons are extremely lightweight and exist in a cloud orbiting the nucleus.
The exterior shell of the atom is not the only place electrons can be found in an atom. Though the electron shell is the only stable location for electrons a heavy atom that undergoes beta decay can emit an electron from its nucleus. To avoid confusion electrons emitted by the nucleus are referred to as beta particles.
Neutrons and protons commonly called nucleons are bound together in the dense inner core of an atom the nucleus where they account for 999 percent of the atoms mass.