Plants absorb carbon dioxide from the atmosphere and animals eat plants. Understand how decay and half life work to enable radiometric dating.
This means all living things have radioactive carbon-14 in them.
Radioactive dating half life. The rule is that a sample is safe when its radioactivity has dropped below detection limits. And that occurs at 10 half-lives. So if radioactive iodine-131 which has a half-life of 8 days is injected into the body to treat thyroid cancer itll be gone in 10 half-lives or 80 days.
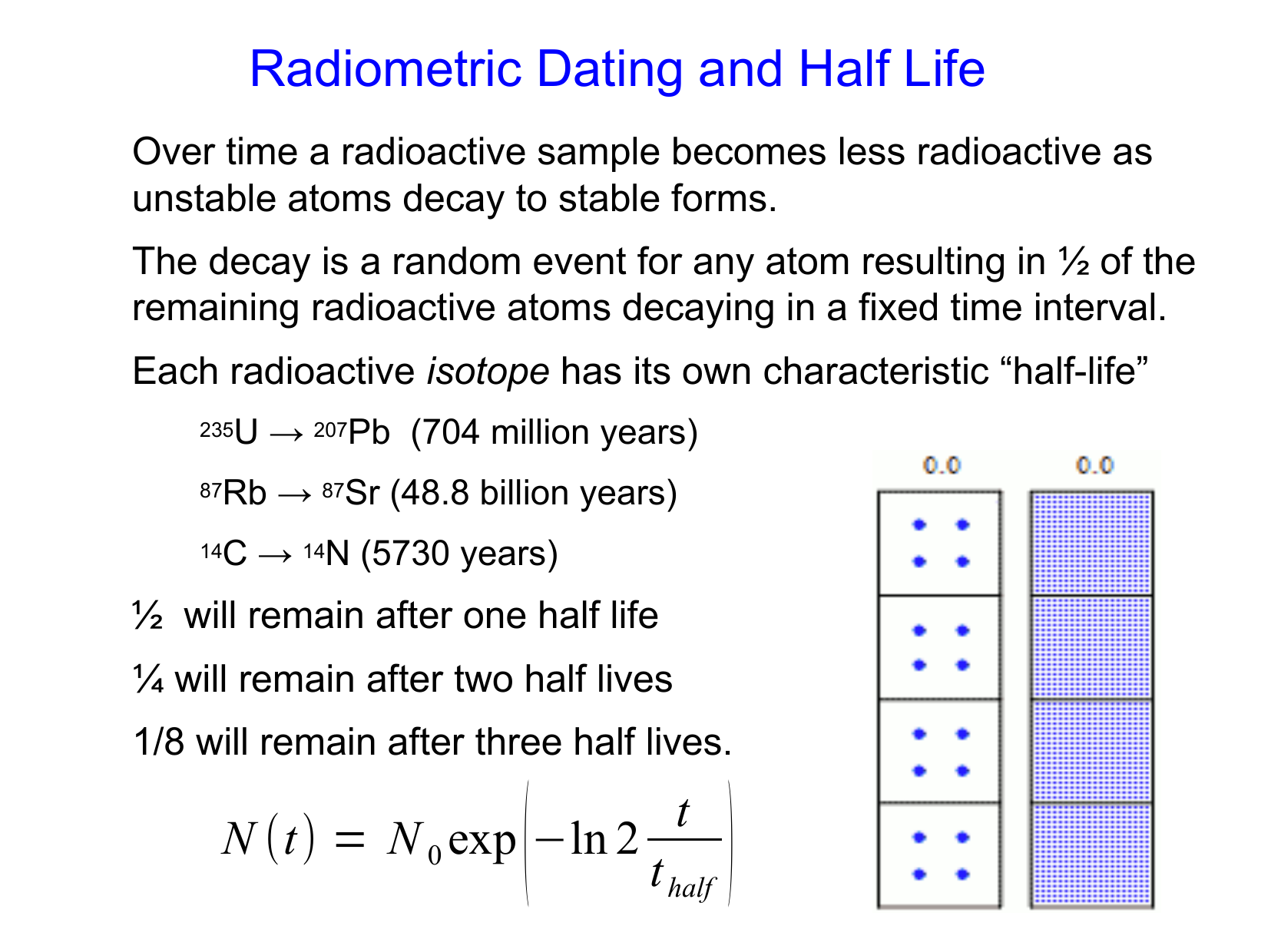
This method of dating specimens is called radioactive dating. Radioisotopes with longer half-lives are used to date older specimens and those with shorter half-lives are used to date younger ones. Carbon-14 dating is used to date specimens younger than about 60000 years old.
Radioactive dating is a process by which the approximate age of an object is determined through the use of certain radioactive nuclides. For example carbon-14 has a half-life of 5730 years and is used to measure the age of organic material. Radiocarbon dating is also simply called carbon-14 dating.
Carbon-14 is a radioactive isotope of carbon with a half-life of 5730 years which is very short compared with the above isotopes and decays into nitrogen. Radioactive Dating Game A. Click the Pause button at the bottom of the window.
Click the Add 10 button below the Bucket o Atoms repeatedly until there are no more atoms left in the bucket. There are now 100 carbon-14 atoms on the screen. The half-life of carbon-14 is about 5700 years.
Using the half-life it is possible to predict the amount of radioactive material that will remain after a given amount of time. C -14 dating procedures have been used to determine the age of organic artifacts. Its half-life is approximately 5700 years.
Understand how decay and half life work to enable radiometric dating. Play a game that tests your ability to match the percentage of the dating element that remains to the age of the object. Sample Learning Goals Explain the concept of half-life including the random nature of it in terms of single particles and larger samples.
An important method of radioactive dating is carbon-14 dating. Carbon-14 nuclei are produced when high-energy solar radiation strikes 14 N nuclei in the upper atmosphere and subsequently decay with a half-life of 5730 years. 1558 The half-life for the radioactive decay of C-14 is 5730 years.
How long will it take for 25 of the C-14 atoms in a sample of C-14 to decay. If a sample of C-14 initially contains 19 mmol of. You can find the half-life of a radioactive element using the formula.
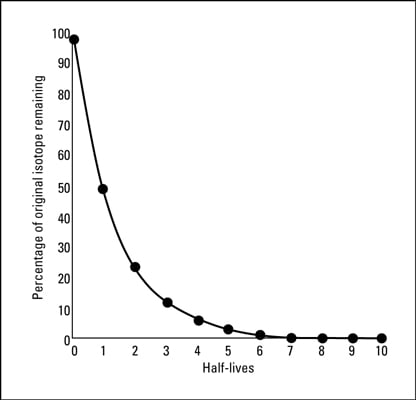
Where t12 is the half-life of the particle t is the elapsed time N0 is the quantity in the beginning and Nt is the quantity at time t. This equation is used in the calculator when solving for half-life time. Radioactive elements decay that is change into other elements by half lives If a half life is equal to one year then one half of the radioactive element will have decayed in the first year after the mineral was formed.
One half of the remainder will decay in the next year leaving one-fourth remaining and so forth. The answer can be found by examining Figure 2227 which shows how the number of radioactive nuclei in a sample decreases with time. The time in which half of the original number of nuclei decay is defined as the half-life t 1 2.
After one half-life passes half of the remaining nuclei will decay in the next half-life. Systems that have been exploited for radiometric dating have half-lives ranging from only about 10 years eg tritium to over 100 billion years eg Samarium-147. However in general the half-life of a nuclide depends solely on its nuclear properties and is essentially a constant.
Also the half-life of potassium-40 is only 13 billion years so it can be used to date rocks as young as 50000 years old. In 1947 a radioactive dating method for determining the age of organic materials was developed by Willard Frank Libby who received the Nobel Prize in chemistry in 1960 for his radiocarbon research. The best radioactive element to use to date human fossils is Carbon-14.
There are several reasons why but the main reasons is that Carbon-14 is a naturally occurring isotope in all forms of life and its half-life is about 5730 years so we are able to use it to date more recent forms of life relative to the geologic time scale. The half-life of the uranium-238 to lead-206 is 447 billion years. The uranium-235 to lead-207 decay series is marked by a half-life of 704 million years.
These differing rates of decay help make. Carbon-dating uses the half-life of Carbon-14 to find the approximate age of an object that is 40000 years old or younger. Radiographers use half-life information to make adjustments in the film exposure time due to the changes in radiation intensity that occurs as radioisotopes degrade.
Plants absorb carbon dioxide from the atmosphere and animals eat plants. This means all living things have radioactive carbon-14 in them. When an organism eg a tree dies it stops taking in carbon.