When a substance loses an electron its oxidation state increases. For example for the redox reaction ceH_2 F_2 2 HF nonumber.

Redox reactions reactions in which theres a simultaneous transfer of electrons from one chemical species to another are really composed of two different reactions.
Redox reactions for dummies. Redox reactions reactions in which theres a simultaneous transfer of electrons from one chemical species to another are really composed of two different reactions. Oxidation a loss of electrons and reduction a gain of electrons. The electrons that are lost in the oxidation reaction are the same electrons that are gained in the reduction.
This is an introduction to oxidation reduction reactions which are often called redox reactions for short. An oxidation reduction redox reaction happens w. In acid solutions take the number of oxygen atoms needed and add that same number of water molecules to the side that needs oxygen.
In basic solutions add. To the side that needs oxygen for every oxygen atom that is needed. Then to the other side of the equation add half as many water molecules as.
Redox reactions are oxidation-reduction chemical reactions in which the reactants undergo a change in their oxidation states. The term redox is a short form of reduction-oxidation. All the redox reactions can be broken down into two different processes a reduction process and an oxidation process.
All the magic that we know is in the transfer of electrons. Reduction gaining electrons and oxidation the loss of electrons combine to form Redox chemist. Redox reactions are reactions in which one species is reduced and another is oxidized.
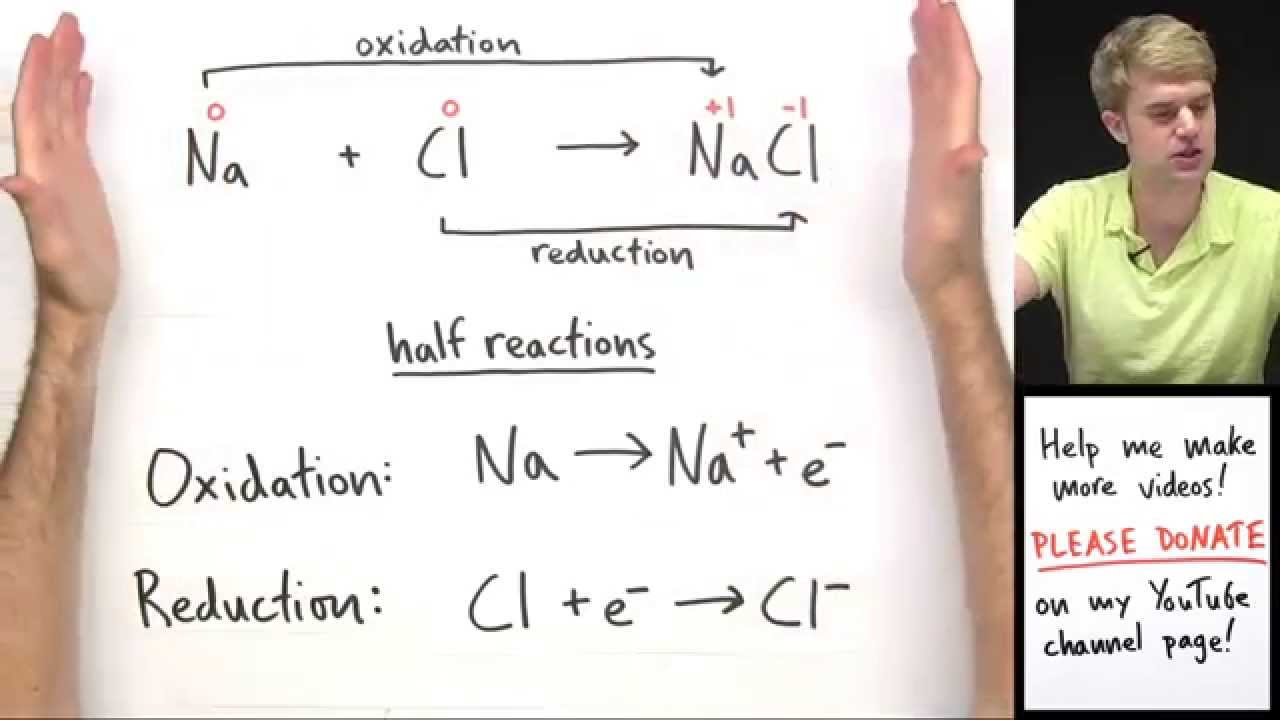
Therefore the oxidation state of the species involved must change. These reactions are important for a number of applications including energy storage devices batteries photographic processing and energy production and utilization in living systems including humans. The redox reaction creates two ions with opposite charges that are attracted to one another and create sodium chloride or table salt.
At this point its also important to discuss reducing and. Oxidation and Reduction Reacions. Reduction and oxidation occurs whenever there is a change in the amount of electrons.
When an element gains electrons it is being reduced. If an element loses electrons then it is oxidized. Neither of these will happen by themselves.
A redox reaction is a reaction that involves a change in oxidation state of one or more elements. When a substance loses an electron its oxidation state increases. Thus it is oxidized.
When a substance gains an electron its oxidation state decreases thus being reduced. For example for the redox reaction ceH_2 F_2 2 HF nonumber. Redox reactions or reduction-oxidation reactions are reactions in which electrons are exchanged.
The preceding reactions are examples of other types of reactions such as combination combustion and single-replacement reactions but theyre all redox reactions. They all involve the transfer of electrons from one chemical species to another. Oxidation-reduction reactions are vital for biochemical reactions and industrial processes as well.
Redox reactions are used to reduce ores to obtain metals to produce electrochemical cells to convert ammonia into nitric acid for fertilizers and to coat compact discs. Combustion reactions of fuels and foods are types of redox reactions that are essential for life and civilization because heat is the most important product of these reactions. Following are common examples of combustion reactions.
The burning of coal wood natural gas and petroleum heats our homes and provides the majority of our electricity. What is a redox reaction simple. An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species.
An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron. Redox reactions reactions in which theres a simultaneous transfer of electrons from one chemical species to another are really composed of two different reactions. Oxidation a loss of electrons and reduction a gain of electrons.
These reactions are coupled as the electrons that are lost in the oxidation reaction are the same electrons that are gained in the reduction reaction. Using the Nernst equation to calculate the cell potential when concentrations are not standard conditions. Watch the next lesson.
Learn how to use the half-reaction method to balance equations for redox reactions occurring in acidic and basic solution. Learn how to use the half-reaction method to balance equations for redox reactions occurring in acidic and basic solution. If youre seeing this message it means were having trouble loading external resources on our.
Introducing oxidation states oxidation and reduction. Some tips for remembering oxidation and reduction. Watch the next lesson.
The chemical reactions in which oxidation and reduction occur simultaneously are called oxidation-reduction reactions ORR or redox reactions. In terms of electron transfer a redox reaction is defined as the process in which electrons are transferred from one substance reducing agent to another oxidizing agent.