Sugar is a pure substance because its composition is homogeneous throughout. Will it be a pure substance or a mixture.

Milk is not a pure substance.
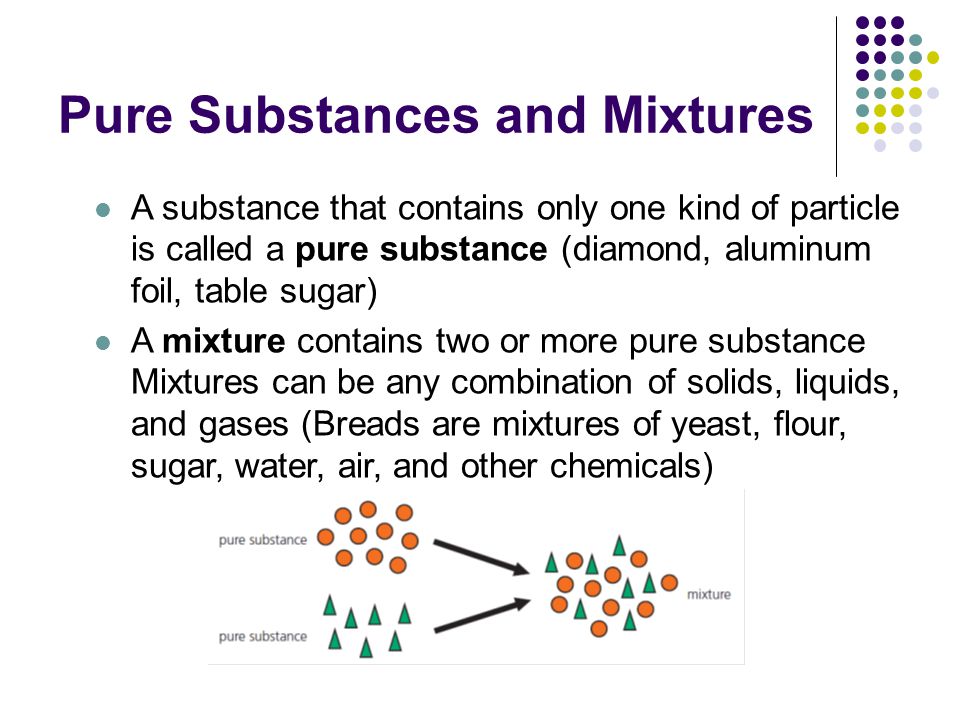
Sugar pure substance or mixture. Sugar is a pure substance because its composition is homogeneous throughout. Pure substances can be either elements or compounds. Sugar or sucrose is a compound with the chemical formula C 12 H 22 O 11.
Compounds consist of two or more elements that have a specific ratio. Sugar mixture or pure substance. When a plant takes in carbon dioxide from the air and energy from the sun it forms sugar in its purest form.
Compounds consist of two or more elements that have a specific ratio. Sugar is a pure substance because its composition is homogeneous throughout. Table sugar added to a cup of coffee and stirred.
The air we breathe non polluted Mixture. Mixtures vs Pure substance. Classification of matter worksheet 1.
A pure substance has a definite and constant composition like salt or sugar. A pure substance can be either an element or a compound but the composition of a pure substance doesnt vary. Sucrose sugar crystals obtained from sugarcane and beetroot are mixed together.
Will it be a pure substance or a mixture. Give reasons for the same. It will be a pure substance.
It is because sugar obtained by different sources like sugarcane and beetroot will have the same composition. Related questions 0. A mixture on the other hand is the physical combination of two or more substances.
In this article we are going to focus on pure substances. In chemistry the pure substance is a very simple concept to grasp. Its a substance made of only one type of atom or only one type of molecule a cluster of atoms bonded together.
A pure substance. More specifically each molecule of sugar has 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms. Other forms of sugar like glucose fructose and lactose are also made up of carbon hydrogen and oxygen but they have different amounts of the three elements.
What Is an Element. Table sugar or sucrose is not a mixture. It is a compound.
Let me start with a pure substance and work up from there. Elements are pure substances because they cannot be broken down any more. It is a mixture composed of the cereal part and the awesome marshmallow part.
Each element can be identified and separated from the other through physical means. Sugar can be mixed into all kinds. Sugar can be mixed into all kinds of things to create a mixture or a solution but the sugar itself is a compound.
Milk is not a pure substance. It is considered a mixture because it does not occur naturally on its own. It is a mixture that combines mostly water sugar fat and proteins.
A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves as a single substance. Another word for a homogeneous mixture is solutionThus a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. Pure Substance is either element or compound.
It contains the same kind of atom or molecules and has a definite set of physical and chemical properties b Impure substance. A substance in which some other substances are also present in smaller or larger amounts is called an impure substance. Mixtures are impure substance.
Pure Substance vs Mixture The difference between pure substance and mixture is that the pure substance consists of one kind of component present in the definite composition whereas the mixture is the substance that consists of more than one component which does not exist in the fixed composition. Pure Substance or Mixture Element Compound Homogeneous Heterogeneous concrete Mixture Heterogeneous sugar pure water C 12H 22O 11 H 2O Mixture Homogeneous iron filings Fe Pure Substance Element limestone CaCO 3 Pure Substance Compound orange juice wpulp Mixture Heterogeneous. Examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate.
Depending on who you talk to homogeneous mixtures may be considered examples of pure substances. Table sugar Pure substance Compound 8. Table sugar added to a cup of coffee and stirred Mixture Homgenous mixture 9.
Kool-aid is added to water Mixture Homogenous mixture 10. Coca-cola Mixture Homogenous mixture 11. Helium gas used to inflate a balloon Pure Substance Element 12.
Mercury metal used in old thermometers Pure substance Element. Pure substance Cu mercury. Pure substance Hg aluminium.
Pure substance Al iron. Pure Substance or Mixture. Pure Substance and Mixture Examples 30 Terms.
Properties of Matter 15 Terms.