
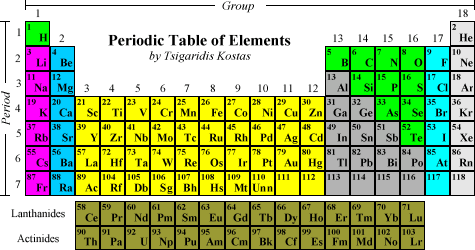
A vertical column of elements in the periodic table is called a group. The elements are arranged in order of increasing atomic number the horizontal rows are called periods the vertical columns are.
The horizontal rows of elements in the periodic table are called periods therefore the answer is B.
The horizontal rows of the periodic table are called. On the periodic table the seven horizontal rows are called periods. On the left-hand side of the periodic table the row numbers are given as one through seven. Moving across a period from left to right the atomic number of the elements increases.
Rows six and seven contain elements called transition elements which are subdivided into the. According to the Aufbau principle the order of filling atomic orbitals is 1s 2s 2p 3s 3p and so on. The elements in a modern periodic table are arranged in an increasing order of their atomic numbers.
In the modern periodic table the horizontal rows are known as periods and vertical columns are known as groups. The horizontal rows of elements in the periodic table are called periods therefore the answer is B. The periods are numbered 1 through 7.
Horizontal rows in the periodic table are called periods. Also access detailed answers to various other Science Maths questions for free. The horizontal rows in the periodic table are periods while the vertical rows are called groups.
Answered 2 years ago Horizontal rows in periodic table are called periods they are seven in numbers elements in same period may not have similar quality. While vertical rows in periodic table are called columns or family. 16K views View 2 Upvoters.
A vertical column of elements in the periodic table is called a group. A horizontal row of elements in the periodic table is called a period. Atoms of elements in the same group have the same number of valence electrons.
As you move from left to right on the periodic table the atomic number increases. The modern periodic table is based closely on the ideas he used. The elements are arranged in order of increasing atomic number the horizontal rows are called periods the vertical columns are.
How many horizontal rows are there in the modern periodic table and what are they called. Asked Jun 13 2018 in Class X Science by aditya23 -2145 points 0 votes. Horizontal rows on a periodic table are called periods.
Vertical columns on a periodic table are called groups. The Horizontal Rows Of Elements In Periodic Table Are Called. The Horizontal Rows Of Elements In Periodic Table Are Called Quizlet.
The Horizontal Rows Of Elements In Periodic Table Are Called Brainly. The Horizontal Rows Of Elements In Periodic Table Are Called Select One. What are the horizontal rows called in the periodic table.
Closer to farther from. Electrons that are closer tofarther from the nucleus have less energy than those electrons that are closer tofarther from the nucleus. Periodic table consist of horizontal rows and vertical column.
The horizontal rows are called period while vertical column are called groups. There are 7 periods and 18 column in modern periodic table. Here we will discuss some trends along period and groups.
As we move from left to right across the periodic table the number. The horizontal rows on the periodic table of the elements are called periods. Every element in a period has the same number of atomic orbitals.
For instance hydrogen and helium are in the first period so they both have electrons in one orbital. The modern periodic table consists of horizontal rows called periods and vertical columns called groups. These are discussed below.
Groups in Modern Periodic Table A group may be defined as vertical column in the periodic table. The horizontal rows and vertical columns of a periodic table are called ____and ___ respectively. W Norton Compan The Modern Periodic Table Periodic table Horizontal rows are from CHEM 114 at University of Saskatchewan.
The Group 7 elements fluorine F chlorine Cl bromine Br iodine I and astatine At have seven electrons in the outer shell. Group 0 elements helium He neon Ne argon Ar. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.