The two main types of chemical bonds are ionic and covalent bonds. Polar covalent bonds and nonpolar covalent bonds.

There are two major types of covalent bonds.
Types of covalent bonds. Non-polar covalent bond During the Formation of a covalent bond between two atoms which belong to the same element the pair which is shared will lie in the middle of the two atoms. This means the atoms involved in sharing will share the electrons equally. Hence molecule obtained will be electrically symmetrical.
Covalent bonds and ionic bonds are types of atomic bonds. These bonds are different in their properties and structure. The covalent bonds include pairs of electrons by two atoms binding them in a fixed orientation.
While a bond between two ions is called ionic bonds. Covalent bonds types The covalent bond has three types of covalent bond which are single covalent bond double covalent bond and triple covalent bond. A covalent bond is a chemical bond that involves the sharing of electron pairs between two atoms.
These electron pairs are known as bonding electron pairs and they share these electrons to form covalent bond. This bonding is primarily found between nonmetals. However it can also be observed between nonmetals and metals.
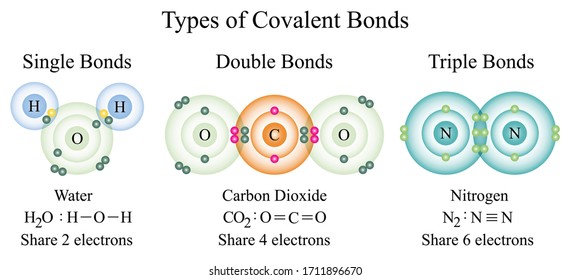
When three pairs of electrons are shared between the two participating atoms a triple bond is formed. Triple covalent bonds are represented by three dashes. These are the least stable types of covalent bonds.
A covalent bond formed between two different atoms is known as Polar covalent bond. For example when a Covalent bond is formed between H and Cl it is polar in nature because Cl is more electronegative than H atom. Therefore electron cloud is shifted towards Cl atom.
The types of covalent bonds can be distinguished by looking at the Lewis dot structure of the molecule. For each molecule there are different names for pairs of electrons depending if it is shared or not. A pair of electrons that is shared between two atoms is called a bond pair.
A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. For many molecules the sharing of electrons allows each atom to attain the equivalent of a full outer shell. Types Of Covalent Bonds Covalent bonds can be divided into two main categories polar covalent and non-polar covalent.
Whether or not two atoms will form a polar or non-polar covalent bond depends on those atoms respective electronegativities. Covalent bonds Silicon carbon germanium and a few other elements form covalently bonded solids. In these elements there are four electrons in the outer.
A brief treatment of covalent bonds follows. The three types as mentioned in the other answers are polar covalent nonpolar covalent and coordinate covalent. The first polar covalent is formed between two nonmetals that have a difference in electronegativity.
They share their electron density unevenly. Covalent bonds involve two atoms typically nonmetals that share electron density to form strong bonding interactions. Covalent bonds include single double and triple bonds and are composed of sigma and pi bonding interactions where 2 4 or 6 electrons are shared respectively.
In covalent bonding overlapping depends on the fact that which type of molecule is going to form and which type of covalent bonding is being done by it which means either only sigma bonding like saturated hydrocarbon methane ethane etc or sigma and pi bonding like unsaturated hydrocarbon ethylene acetylene etc. The two main types of chemical bonds are ionic and covalent bonds. An ionic bond essentially donates an electron to the other atom participating in the bond while electrons in a covalent bond are shared equally between the atoms.
The only pure covalent bonds occur between identical atoms. A covalent bond is formed when two atoms share a pair of electrons. Covalent bonding occurs in most non-metal elements and in compounds formed between non-metals.
These shared electrons are found. Types of Covalent Bonds. Polar and Nonpolar.
Electrons are shared differently in ionic and covalent bonds. Covalent bonds can be non-polar or polar and react to electrostatic charges. Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na and negative charged Cl- ions.
There are two major types of covalent bonds. Polar covalent bonds and nonpolar covalent bonds. Polar covalent bonds exist between two atoms with a difference between their electronegativity values in the range of 04 to 17.
Nonpolar covalent bonds form if this difference is lower than 04.