The elements silicon germanium arsenic antimony tellurium etc. All of which are the basic building blocks of organic compounds.

Gases such as oxygen and solids such as carbon.
Which elements are nonmetals. Even though there are only 7 elements within the nonmetals group two of these elements hydrogen and helium make up about 98 of the mass of the universe. Nonmetals form more compounds than metals. Living organisms consist mainly of nonmetals.
The nonmetal element group consists of hydrogen carbon nitrogen oxygen phosphorus sulfur and selenium. Hydrogen acts as a nonmetal at normal temperatures and pressure and is generally accepted to be part of the nonmetal group. Non-metals are natural materials that do not produce heat or electricity and that are structurally brittle can not be easily rolling moulding extruding or pressing.
Chemically hydrogen carbon nitrogen oxygen phosphorus arsenic and selenium are the non-metallic elements in the periodic table. What is called ductility. In chemistry a nonmetal or non-metal is a chemical element that mostly lacks the characteristics of a metalPhysically a nonmetal tends to have a relatively low melting point boiling point and densityA nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivityChemically nonmetals tend to have relatively high ionization energy.
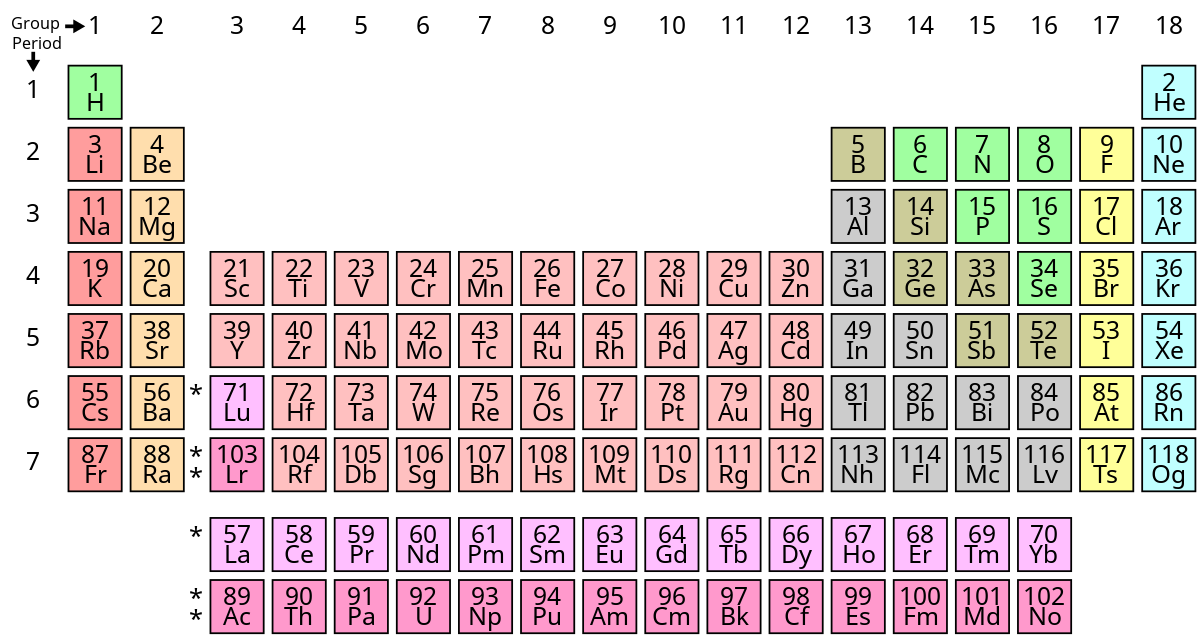
Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. The halogens and noble gases are nonmetals but the nonmetal element group usually consists of the following elements. What elements are nonmetals.
The elements that are generally considered other nonmetals include hydrogen carbon nitrogen phosphorus oxygen sulfur and selenium. Nitrogen and phosphorus are included in the subgroup pnictogens. The non-metals exist in two of the three states of matter at room temperature.
Gases such as oxygen and solids such as carbon. The non-metals have no metallic luster and do not reflect light. They have oxidation numbers of 4 -3 and -2.
The Non-Metal elements are. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions. Some nonmetals are liquids.
These elements are shown in the following figure. The nonmetals in the periodic table. Plastic is a compound of nonmetallic elements so its properties make it a popular coating for electric wires to prevent the current from escaping.
Nonmetals are defined as elements that cannot conduct _____. There are fewer nonmetals than there are elements in any other class. Properties of nonmetals include a high boiling point.
B ability to conduct heat. D none of the above. Although five times more elements are metals than nonmetals two of the nonmetalshydrogen and heliummake up over 99 per cent of the observable Universe and oneoxygenmakes up close to half of the Earths crust oceans and atmosphere.
Examples of non-metals include carbon oxygen nitrogen and hydrogen. All of which are the basic building blocks of organic compounds. Compared to metals nonmetals display a highly variable range of properties in terms of their atomic and chemical behavior.
Nonmetals are elements that generally cannot conduct electricity. They are the second largest class of elements after metals. Examples of nonmetals include hydrogen carbon chlorine and helium.
Properties of nonmetals include a relatively low boiling point so many nonmetals are gases. Nonmetals with the exception of hydrogen are located on the right side of the periodic table. Elements that are nonmetals are hydrogen carbon nitrogen phosphorus oxygen sulfur selenium all of the halogens and the noble gases.
Some metals appear coloured Cu Cs Au have low densities eg. Be Al or very high melting points eg. W Nb are liquids at or near room temperature eg.
Hg Ga are brittle eg. Os Bi not easily machined eg. Ti Re or are noble hard to oxidise eg.
Au Pt or have nonmetallic structures Mn and Ga are structurally analogous to respectively white P and I. Metals are substances that tend to donate electrons. They are known to be lustrous or shiny highly malleable or ductile and are very good conductors of electricity and heat.
The elements silicon germanium arsenic antimony tellurium etc. Show properties characteristic of both metals and non-metals. These are called metalloids or semimetals.
These are present diagonally on the p block of modern periodic table. In chemistry a nonmetal or non-metal is a chemical element that mostly lacks metallic attributesPhysically nonmetals tend to be highly volatile easily vaporized have low elasticity and are good insulators of heat and electricity. Chemically they tend to have high ionization energy and electronegativity values and gain or share electrons when they react with other elements or compounds.